Abstract
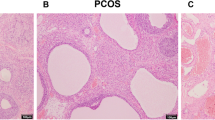
Polycystic ovary syndrome (PCOS), a common female endocrinopathy associated with both reproductive and metabolic disorders, has an unclear etiology and unsatisfactory management methods. Carboxypeptidase X, M14 family member 1 (CPXM1) is a protein involved in follicular atresia, insulin production, and adipose tissue production, though its role in PCOS is not fully understood. We used a 60% high-fat diet (HFD) plus dehydroepiandrosterone (DHEA)-induced PCOS mouse model to determine the role of CPXM1 in abnormal glucose metabolism and ovarian dysfunction in PCOS. We found that serum CPXM1 concentrations were higher in PCOS mice and positively correlated with increased levels of serum testosterone and insulin. In both ovarian and adipose tissues of PCOS mice, CPXM1 mRNA and protein levels were significantly increased but GLUT4 levels were significantly decreased. Immunohistochemistry (IHC) staining of the ovary showed increased CPXM1 expression in PCOS. In addition, the protein expression of phosphorylated protein kinase B (p-Akt) was also significantly decreased in PCOS mice. Furthermore, mRNA levels of inflammatory markers such as TNF-α, IL-6, IFN-α, and IFN-γ were increased in ovarian and adipose tissues of PCOS mice. However, IRS-1, IRS-2, and INSR levels were significantly decreased. Our results indicated for the first time that abnormally high expression of CPXM1, increased adiposity, impaired glucose tolerance, and chronic low-grade inflammation may act together in a vicious cycle in the pathophysiology of PCOS. Our research suggests the possibility of CPXM1 as a potential therapeutic target for the treatment of PCOS.













Similar content being viewed by others
Data availability
The datasets used and/or generated during this research are available upon valid question from the corresponding author.
Code availability
Not applicable.
Change history
07 July 2022
A Correction to this paper has been published: https://doi.org/10.1007/s43032-022-01021-x
References
Chen Y, Yang T, Hao C, Zhao J. A retrospective study of letrozole treatment prior to human chorionic gonadotropin in women with polycystic ovary syndrome undergoing in vitro fertilization at risk of ovarian hyperstimulation syndrome. Med Sci Monit. 2018;24:4248–53. https://doi.org/10.12659/MSM.910743.
Hamilton KP, Zelig R, Parker AR, Haggag A. Insulin resistance and serum magnesium concentrations among women with polycystic ovary syndrome. Curr Dev Nutr. 2019;3:1–12. https://doi.org/10.1093/cdn/nzz108.
Siklar Z, Berberoğlu M, Çamtosun E, Kocaay P. Diagnostic characteristics and metabolic risk factors of cases with polycystic ovary syndrome during adolescence. J Pediatr Adolesc Gynecol. Elsevier Ltd. 2015;28:78–83. https://doi.org/10.1016/j.jpag.2014.05.006.
Harris HR, Terry KL. Polycystic ovary syndrome and risk of endometrial, ovarian, and breast cancer: a systematic review. Fertil Res Pract. 2016;2:1–9. https://doi.org/10.1186/s40738-016-0029-2.
Qin L, Huang CC, Yan XM, Wang Y, Li ZY, Wei XC. Long non-coding RNA h19 is associated with polycystic ovary syndrome in Chinese women: a preliminary study. Endocr J. 2019;66:587–95. https://doi.org/10.1507/endocrj.EJ19-0004.
Wang M, Zhao D, Xu L, Guo W, Nie L, Lei Y, et al. Role of PCSK9 in lipid metabolic disorders and ovarian dysfunction in polycystic ovary syndrome. Metabolism. Elsevier Inc. 2019;94:47–58. Available from: https://doi.org/10.1016/j.metabol.2019.02.002.
He FF, Li YM. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: a review. J Ovarian Res. 2020;13:1–13. https://doi.org/10.1186/s13048-020-00670-3.
Mannerås-holm L, Leonhardt H, Kullberg J, Jennische E, Lo L, Stener-victorin E, et al. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab. 2011;96:304–11. https://doi.org/10.1210/jc.2010-1290.
Li Y, Zheng Q, Sun D, Cui X, Chen S, Bulbul A, et al. Dehydroepiandrosterone stimulates inflammation and impairs ovarian functions of polycystic ovary syndrome. J Cell Physiol. 2019;234:7435–47. https://doi.org/10.1002/jcp.27501.
Wang J, Wu D, Guo H, Li M. Hyperandrogenemia and insulin resistance: the chief culprit of polycystic ovary syndrome. Life Sci. Elsevier Inc. 2019;236:116940. https://doi.org/10.1016/j.lfs.2019.116940.
Sapio MR, Fricker LD. Carboxypeptidases in disease: insights from peptidome studies. Proteomics Clin Appl. 2014;8:327–37. https://doi.org/10.1002/prca.201300090.
Kim YH, Barclay JL, He J, Luo X, O’Neill HM, Keshvari S, et al. Identification of carboxypeptidase X (CPX)-1 as a positive regulator of adipogenesis. FASEB J. 2016;30:2528–40. https://doi.org/10.1096/fj.201500107R.
Kim Y, Neill HMO, Whitehead JP. Glycoprotein. Biochem Biophys Res Commun. Elsevier Ltd. 2015;468:894–9. https://doi.org/10.1016/j.bbrc.2015.11.053.
Li PF, Meng JZ, Jing JJ, Bi XL, Wang K, Zhu ZW, Lü LH. Follicular development related genes screening and differential expressed analysis by transcriptome sequencing in bovine ovary. Sci Agric Si. 2018;51:15:3000–3008. http://www.chinaagrisci.com/EN/10.386.
Zheng M, Long J, Chelariu-Raicu A, Mullikin H, Vilsmaier T, Vattai A, et al. Identification of a novel tumor microenvironment prognostic signature for advanced-stage serous ovarian cancer. Cancers (Basel). 2021;13(13):3343. https://doi.org/10.3390/cancers13133343.
Cai S, Yu X, Gu Z, Yang Q, Wen B, Sheng J, et al. A 10-gene prognostic methylation signature for stage I–III cervical cancer. Arch Gynecol Obstet. Springer Berlin Heidelberg. 2020;301:1275–87. https://doi.org/10.1007/s00404-020-05524-3
Lai H, Jia X, Yu Q, Zhang C, Qiao J, Guan Y, et al. High-fat diet induces significant metabolic disorders in a mouse model of polycystic ovary Syndrome. Biol Reprod. 2014;91:1–11. https://doi.org/10.1095/biolreprod.114.120063.
Fraulob JC, Ogg-Diamantino R, Fernandes-Santos C, Aguila MB, Mandarim-de-Lacerda CA. A mouse model of metabolic syndrome: insulin resistance, fatty liver and non-alcoholic fatty pancreas disease (NAFPD) in C57BL/6 mice fed a high fat diet. J Clin Biochem Nutr. 2010;46:212–23. https://doi.org/10.3164/jcbn.09-83.
Ullah A, Wang M, Yang J, Appiah E, Czika A, Sah SK, et al. Ovarian inflammatory mRNA profiles of a dehydroepiandrosterone plus high-fat diet-induced polycystic ovary syndrome mouse model. Reprod BioMed Online. 2022;44(5):791–802. https://doi.org/10.1016/j.rbmo.2021.10.024.
Benrick A, Chanclón B, Micallef P, Wu Y, Hadi L, Shelton JM. Adiponectin protects against development of metabolic disturbances in a PCOS mouse model. PNAS. 2017;114:E7187–96. https://doi.org/10.1073/pnas.1708854114.
Xie M, Li M, Zhou J, Ding X, Shao Y, Jing J, et al. Brain-derived neurotrophic factor promotes human granulosa-like tumor cell steroidogenesis and proliferation by activating the FSH receptor-mediated signaling pathway. Sci Rep. Springer US. 2017:1–13. https://doi.org/10.1038/s41598-017-00203-x.
Nishi Y, Yanase T, Mu Y, Oba K, Ichino I, Saito M, et al. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology. 2001;142(1):437–45. https://doi.org/10.1210/endo.142.1.7862.
Abizadeh M, Novin MG, Amidi F, Ziaei SA, Abdollahifar MA. Potential of auraptene in improvement of oocyte maturation, fertilization rate, and inflammation in polycystic ovary syndrome mouse model. Reprod Sci. 2020;27:1742–51.
Ebrahimi F, Rostami S, Nekoonam S, Rashidi Z, Sobhani A, Amidi F. The effect of astaxanthin and metformin on oxidative stress in granulosa cells of BALB C mouse model of polycystic ovary syndrome. Reprod Sci. 2021;28:2807–15.
Chen F, Liao Y, Chen M, Yin H, Chen G, Huang Q, et al. Evaluation of the efficacy of sex hormone–binding globulin in insulin resistance assessment based on HOMA-IR in patients with PCOS. Reprod Sci. 2021;28:2504–13.
Gambineri A, Pelusi C, Vicennati V, Pagotto U, Pasquali R. Obesity and the polycystic ovary syndrome. Int J Obes. 2002;26(7):883–96. https://doi.org/10.1038/sj.ijo.0801994.
Zhang H, Yi M, Zhang Y, Jin H, Zhang W, Yang J, et al. High-fat diets exaggerate endocrine and metabolic phenotypes in a rat model of DHEA-induced PCOS. Repro. 2016;151:431–41. https://doi.org/10.1530/rep-15-0542.
Wu S, Divall S, Nwaopara A, Radovick S, Wondisford F, Ko C, et al. Obesity-induced infertility and hyperandrogenism are corrected by deletion of the insulin receptor in the ovarian theca cell. Diabet. 2014;63:1270–82. https://doi.org/10.2337/db13-1514.
Deligeoroglou E, Vrachnis N, Athanasopoulos N, Iliodromiti Z, Sifakis S, Iliodromiti S. Mediators of chronic inflammation in polycystic ovarian syndrome. Gynecol Endocrinol. 2012;28:974–8. https://doi.org/10.3109/09513590.2012.683082.
Gonzalez F, Thusu K, Abdei-rahman E, Prabhala A, Tomani M, Dandona P, et al. Elevated serum levels of tumor necrosis factor alpha in normal-weight women with polycystic ovary syndrome. Metabol. 1999;48:437–41. https://doi.org/10.1016/S0026-0495(99)90100-2.
Panidis D, Kita M, Katsikis I, Karkanaki A, Karayannis V, Rousso D. Mechanisms of infertility in polycystic ovary syndrome. Aristotle Univ Med J. 2006;33:67–77.
Lin Y, Tsai S, Lin M, Yang C, Huang M, Wu M, et al. Interleukin-6 as an early chronic inflammatory marker in polycystic ovary syndrome with insulin receptor substrate-2 polymorphism. Am J Reprod Immunol. 2011;66:527–33. https://doi.org/10.1111/j.1600-0897.2011.01059.x.
Tumu VR, Govatati S, Guruvaiah P, Deenadayal M, Shivaji S, Bhanoori M. An interleukin-6 gene promoter polymorphism is associated with polycystic ovary syndrome in South Indian women. J Assist Reprod Genet. 2013;30(12):1541–6. https://doi.org/10.1007/s10815-013-0111-1.
Oróstica L, Poblete C, Romero C, Vega M. Pro-inflammatory markers negatively regulate IRS1 in endometrial cells and endometrium from women with obesity and PCOS. Reprod Sci. 2020;27:290–300.
Qin L, Xu W, Li X, Meng W, Hu L, Luo Z, et al. European Journal of Obstetrics & Gynecology and Reproductive Biology Differential expression profile of immunological cytokines in local ovary in patients with polycystic ovarian syndrome : analysis by flow cytometry. Eur J Obstet Gynecol. Elsevier Ireland Ltd. 2016;197:136–41. https://doi.org/10.1016/j.ejogrb.2015.12.003.
Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulin-related ovarian regulatory system in health and disease. Endocr Rev. 1999;20(4):535–82. https://doi.org/10.1210/edrv.20.4.0374.
Lee H, Kim JY, Park JE, Yoon Y. Induction of fas-mediated apoptosis by interferon- g is dependent on granulosa cell differentiation and follicular maturation in the rat ovary. Dev Reprod. 2016;20:315–29. https://doi.org/10.12717/DR.2016.20.4.315.
Tian Y, Jennings J, Gong Y, Sang Y. Viral infections and interferons in the development of obesity. Biomolecules. 2019;9:726. https://doi.org/10.3390/biom9110726.
Mioni R, Mozzanega B, Granzotto M, Pierobon A, Zuliani L, Maffei P, et al. Insulin receptor and glucose transporters mRNA expression throughout the menstrual cycle in human endometrium: a physiological and cyclical condition of tissue insulin resistance. Gynecol Endocrinol. 2012;28:1014–8. https://doi.org/10.3109/09513590.2012.705367.
Cao J, Yu L, Zhao J, Ma H. Effect of dehydroepiandrosterone on the immune function of mice in vivo and in vitro. Mol Immunol. Elsevier. 2019;112:283–90. https://doi.org/10.1016/j.molimm.2019.06.004.
Lehnen AM, Leguisamo NM, Pinto GH, Markoski MM, De Angelis K, Machado UF, et al. The beneficial effects of exercise in rodents are preserved after detraining: a phenomenon unrelated to GLUT4 expression. Cardiovasc Diabetol. 2010;9:1–8. https://doi.org/10.1186/1475-2840-9-67.
Pessin JE, Saltiel AR, Pessin JE, Saltiel AR. Signaling pathways in insulin action: molecular targets of insulin resistance Find the latest version: On diabetes: insulin resistance Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Invest. 2000;106:165–9. https://doi.org/10.1172/JCI10582.
Virkamäki A, Ueki K, Kahn CR, Virkamäki A, Ueki K, Kahn CR. Protein – protein interaction in insulin signaling and the molecular mechanisms of insulin resistance Find the latest version : Protein – protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. J Clin Invest. 1999;103:931–43. https://doi.org/10.1172/JCI6609.
Zhang N, Liu X, Zhuang L, Liu X, Zhao H, Shan Y, et al. Berberine decreases insulin resistance in a PCOS rats by improving GLUT4 : Dual regulation of the PI3K / AKT and MAPK pathways. Regul Toxicol Pharmacol. Elsevier. 2020;110:104544. https://doi.org/10.1016/j.yrtph.2019.104544.
Qiu HY, Chu YL, Li M, Sun YY, Li HF. Tyrosine phosphorylation and protein expression of insulin receptor substrate-2 in the adipose tissue from patients with polycystic ovary syndrome. Zhonghua fu Chan ke za zhi. 2005;40:116–9.
Burks DJ, De Mora JF, Schubert M, Withers DJ, Myers MG, Towery HH, et al. IRS-2 pathways integrate female reproductionand energy homeostasis. Nature. 2000;407:377–82. https://doi.org/10.1038/35030105.
Kubota N, Tobe K, Terauchi Y, Eto K, Yamauchi T, Suzuki R, et al. Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory-cell hyperplasia. Diabet. 2000;49:1880–9. https://doi.org/10.2337/diabetes.49.11.1880.
Du AJ, Wang J, Sun X, Xu X, Zhang F, Shi Y, et al. Family-based analysis of INSR polymorphisms in Chinese PCOS. Reprod Biomed Online. Elsevier Inc.; 2014. https://doi.org/10.1016/j.rbmo.2014.03.028
Lee E, Oh B, Lee J, et al. A novel single nucleotide polymorphism of INSR gene for polycystic ovary syndrome. Fertil Steril. 2008;89:1213–20. https://doi.org/10.1016/j.fertnstert.2007.05.026.
Zhong X, Jin F, Huang C, Du M, Gao M. DNA methylation of AMHRII and INSR gene is associated with the pathogenesis of polycystic ovary syndrome ( PCOS ). Technol Health Care. 2021;29:11–25. https://doi.org/10.3233/THC-218002.
Xu N, Geller DH, Jones MR, Funari VA, Azziz R, Goodarzi MO. Comprehensive assessment of expression of insulin signaling pathway components in subcutaneous adipose tissue of women with and without polycystic ovary syndrome. J Clin Transl Endocrinol. Elsevier Inc; 2015;2:99–104. https://doi.org/10.1016/j.jcte.2015.06.002.
Jones MR, Brower MA, Xu N, Cui J, Mengesha E. Systems genetics reveals the functional context of PCOS loci and identifies genetic and molecular mechanisms of disease heterogeneity. PLoS Genet. 2015;11(8):e1005455. https://doi.org/10.1371/journal.pgen.1005455.
Huang X, Liu G, Guo J, Su Z. The PI3K / AKT pathway in obesity and type 2 diabetes. Int J Biol Sci. 2018;14:1483–96. https://doi.org/10.7150/ijbs.27173.
Huang J, Zhao J, Geng X, Chu W, Li S, Chen Z, et al. Long non-coding RNA lnc-CCNL1-3: 1 promotes granulosa cell apoptosis and suppresses glucose uptake in women with polycystic ovary syndrome. Mol Ther Nucleic Acid. Elsevier Ltd. 2021;23:614–28. https://doi.org/10.1016/j.omtn.2020.12.008.
Tan M, Cheng Y, Zhong X, Yang D, Jiang S, Ye Y, et al. LNK promotes granulosa cell apoptosis in PCOS via negatively regulating insulin-stimulated AKT-FOXO3 pathway. Aging. 2021;13:4617–33. https://doi.org/10.18632/aging.202421.
Diamanti-kandarakis E, Chatzigeorgiou A, Papageorgiou E, Koundouras D, Koutsilieris M. Advanced glycation end-products and insulin signaling in granulosa cells. Exp Biol Med. 2016;241:1438–45. https://doi.org/10.1177/1535370215584937.
Acknowledgements
We thank Jun-Pu Yang and Antonia Adwoa Otoo for the help provided in the processing of tissues and revising the manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 82171624), the Chongqing Natural Science Foundation (No. cstc2020jcyj-msxmX0294), Science and Technology Project of Chongqing Yuzhong District (No. 20200103), and Scientific Research and Innovation Experiment Project of Chongqing Medical University (SRIEP202106; SRIEP202002).
Author information
Authors and Affiliations
Contributions
Sadaf Pervaz: experiment performance, formal analysis, and preparation of the manuscript. Mei-Jiao Wang: study design, methodology, resources, supervision, review, and editing. Amin Ullah: investigation and formal analysis. Enoch Appiah Adu-Gyamfi: formal analysis, review, and editing. Lamptey Jones: formal analysis, review, and editing. Sanjay Kumar Sah: review and editing. Ying-Xiong Wang: fund acquisition, conceptualization, supervision.
Corresponding authors
Ethics declarations
Ethics Approval
The study was approved by the Animal Ethics Committee of Chongqing Medical University on 6 June 2012 (certification no: SCXK [YU] 20210607).
Consent to Participate
Not applicable.
Consent for Publication
All the authors approved the final version of the manuscript.
Conflict of Interest
The authors declare no competing interests.
Additional information
This article was update to correct Enoch Appiah Adu-Gyamfi's name.
Supplementary Information
Below is the link to the electronic supplementary material.

Supplementary file1
Fig. S1 Vaginal smear examination. a Proestrus stage of mice (round, nucleated epithelial cells). b Estrus (cornified squamous epithelial cells). c Metestrus (round, nucleated epithelial cells, cornified squamous epithelial cells, and leukocytes). d Diestrus (predominance of leukocytes). Black arrows represent the proestrus stage; red arrows show estrus; black, blue, and red arrows show metestrus; blue arrows show diestrus. (PNG 2842 kb)

Supplementary file2
Fig. S2 Correlation of the serum levels of CPXM1 with progesterone, LH, and serum FSH. a-c Correlation of the serum levels of CPXM1 with (a) serum progesterone, (b) serum LH, (c) serum FSH. p = 0.1794, p = 0.1470, p = 0.2426. n = 10 per group. (PNG 205 kb)
Rights and permissions
About this article
Cite this article
Pervaz, S., Ullah, A., Adu-Gyamfi, E.A. et al. Role of CPXM1 in Impaired Glucose Metabolism and Ovarian Dysfunction in Polycystic Ovary Syndrome. Reprod. Sci. 30, 526–543 (2023). https://doi.org/10.1007/s43032-022-00987-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-00987-y