Abstract
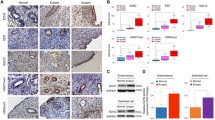
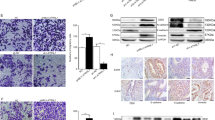
Endometriosis is a disease defined by the presence of abnormal endometrium at ectopic sites, causing pain and infertility in 10% of women. Mutations in the chromatin remodeling protein ARID1A (AT-rich interactive domain-containing protein 1A) have been identified in endometriosis, particularly in the more severe deep infiltrating endometriosis and ovarian endometrioma subtypes. ARID1A has been shown to regulate chromatin at binding sites of the Activator Protein 1 (AP-1) transcription factor, and AP-1 expression has been shown in multiple endometriosis models. Here, we describe a role for AP-1 subunit JUNB in promoting invasive phenotypes in endometriosis. Through a series of knockdown experiments in the 12Z endometriosis cell line, we show that JUNB expression in endometriosis promotes the expression of epithelial-to-mesenchymal transition genes co-regulated by ARID1A including transcription factors SNAI1 and SNAI2, cell adhesion molecules ICAM1 and VCAM1, and extracellular matrix remodelers LOX and LOXL2. In highly invasive ARID1A-deficient endometriotic cells, co-knockdown of JUNB is sufficient to suppress invasion. These results suggest that AP-1 plays an important role in the progression of invasive endometriosis, and that therapeutic inhibition of AP-1 could prevent the occurrence of deep infiltrating endometriosis.






Similar content being viewed by others
Data Availability
The previously published RNA-seq dataset analyzed herein is available at GEO: GSE121198. All other data generated or analyzed during this study are included in this published article.
Code Availability
Not applicable.
References
Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–79.
Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–99.
Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Vigano P. Endometriosis. Nat Rev Dis Primers. 2018;4(1):9.
Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382(13):1244–56.
Bulun SE, Yilmaz BD, Sison C, Miyazaki K, Bernardi L, Liu S, et al. Endometriosis. Endocr Rev. 2019;40(4):1048–79.
Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev. 2014;35(6):851–905.
Mihm M, Gangooly S, Muttukrishna S. The normal menstrual cycle in women. Anim Reprod Sci. 2011;124(3–4):229–36.
Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98(3):511–9.
Bartley J, Julicher A, Hotz B, Mechsner S, Hotz H. Epithelial to mesenchymal transition (EMT) seems to be regulated differently in endometriosis and the endometrium. Arch Gynecol Obstet. 2014;289(4):871–81.
Konrad L, Dietze R, Riaz MA, Scheiner-Bobis G, Behnke J, Horne F, et al. Epithelial-mesenchymal transition in endometriosis-when does it happen? J Clin Med. 2020;9(6):1915.
Liu X, Zhang Q, Guo SW. Histological and immunohistochemical characterization of the similarity and difference between ovarian endometriomas and deep infiltrating endometriosis. Reprod Sci. 2018;25(3):329–40.
Wilson MR, Reske JJ, Holladay J, Wilber GE, Rhodes M, Koeman J, et al. ARID1A and PI3-kinase pathway mutations in the endometrium drive epithelial transdifferentiation and collective invasion. Nat Commun. 2019;10(1):3554.
Wilson MR, Holladay J, Chandler RL. A mouse model of endometriosis mimicking the natural spread of invasive endometrium. Hum Reprod. 2020;35(1):58–69.
Samartzis EP, Samartzis N, Noske A, Fedier A, Caduff R, Dedes KJ, et al. Loss of ARID1A/BAF250a-expression in endometriosis: a biomarker for risk of carcinogenic transformation? Mod Pathol. 2012;25(6):885–92.
Jones S, Wang TL, Shih Ie M, Mao TL, Nakayama K, Roden R, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330(6001):228–231.
Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363(16):1532–43.
Guan B, Mao TL, Panuganti PK, Kuhn E, Kurman RJ, Maeda D, et al. Mutation and loss of expression of ARID1A in uterine low-grade endometrioid carcinoma. Am J Surg Pathol. 2011;35(5):625–32.
Wiegand KC, Lee AF, Al-Agha OM, Chow C, Kalloger SE, Scott DW, et al. Loss of BAF250a (ARID1A) is frequent in high-grade endometrial carcinomas. J Pathol. 2011;224(3):328–33.
Mao TL, Ardighieri L, Ayhan A, Kuo KT, Wu CH, Wang TL, et al. Loss of ARID1A expression correlates with stages of tumor progression in uterine endometrioid carcinoma. Am J Surg Pathol. 2013;37(9):1342–8.
Moore L, Leongamornlert D, Coorens THH, Sanders MA, Ellis P, Dentro SC, et al. The mutational landscape of normal human endometrial epithelium. Nature. 2020;580(7805):640–6.
Suda K, Nakaoka H, Yoshihara K, Ishiguro T, Tamura R, Mori Y, et al. Clonal expansion and diversification of cancer-associated mutations in endometriosis and normal endometrium. Cell Rep. 2018;24(7):1777–89.
Lac V, Nazeran TM, Tessier-Cloutier B, Aguirre-Hernandez R, Albert A, Lum A, et al. Oncogenic mutations in histologically normal endometrium: the new normal? J Pathol. 2019;249(2):173–181.
Lac V, Verhoef L, Aguirre-Hernandez R, Nazeran TM, Tessier-Cloutier B, Praetorius T, et al. Iatrogenic endometriosis harbors somatic cancer-driver mutations. Hum Reprod. 2019;34(1):69–78.
Anglesio MS, Papadopoulos N, Ayhan A, Nazeran TM, Noe M, Horlings HM, et al. Cancer-associated mutations in endometriosis without cancer. N Engl J Med. 2017;376(19):1835–48.
Borrelli GM, Abrao MS, Taube ET, Darb-Esfahani S, Kohler C, Chiantera V, et al. (Partial) Loss of BAF250a (ARID1A) in rectovaginal deep-infiltrating endometriosis, endometriomas and involved pelvic sentinel lymph nodes. Mol Hum Reprod. 2016;22(5):329–37.
Wilson MR, Reske JJ, Holladay J, Neupane S, Ngo J, Cuthrell N, et al. ARID1A mutations promote P300-dependent endometrial invasion through super-enhancer hyperacetylation. Cell Rep. 2020;33(6):108366.
Reske JJ, Wilson MR, Holladay J, Siwicki RA, Skalski H, Harkins S, et al. Co-existing TP53 and ARID1A mutations promote aggressive endometrial tumorigenesis. PLoS Genet. 2021;17(12):e1009986.
Reske JJ, Wilson MR, Holladay J, Wegener M, Adams M, Chandler RL. SWI/SNF inactivation in the endometrial epithelium leads to loss of epithelial integrity. Hum Mol Genet. 2020;29(20):3412–30.
Reske JJ, Wilson MR, Chandler RL. ATAC-seq normalization method can significantly affect differential accessibility analysis and interpretation. Epigenetics Chromatin. 2020;13(1):22.
Wilson MR, Reske JJ, Koeman J, Adams M, Joshi NR, Fazleabas AT, et al. SWI/SNF antagonism of PRC2 mediates estrogen-induced progesterone receptor expression. Cells. 2022;11(6):1000.
Kim JJ, Chapman-Davis E. Role of progesterone in endometrial cancer. Semin Reprod Med. 2010;28(1):81–90.
Nunobiki O, Taniguchi E, Ishii A, Tang W, Utsunomiya H, Nakamura Y, et al. Significance of hormone receptor status and tumor vessels in normal, hyperplastic and neoplastic endometrium. Pathol Int. 2003;53(12):846–52.
Flores VA, Vanhie A, Dang T, Taylor HS. Progesterone receptor status predicts response to progestin therapy in endometriosis. J Clin Endocrinol Metab. 2018;103(12):4561–8.
Trop-Steinberg S, Azar Y. AP-1 Expression and its clinical relevance in immune disorders and cancer. Am J Med Sci. 2017;353(5):474–83.
Ozanne BW, Spence HJ, McGarry LC, Hennigan RF. Transcription factors control invasion: AP-1 the first among equals. Oncogene. 2007;26(1):1–10.
Shaulian E. AP-1–The Jun proteins: oncogenes or tumor suppressors in disguise? Cell Signal. 2010;22(6):894–9.
Ito T, Yamauchi M, Nishina M, Yamamichi N, Mizutani T, Ui M, et al. Identification of SWI.SNF complex subunit BAF60a as a determinant of the transactivation potential of Fos/Jun dimers. J Biol Chem. 2001;276(4):2852–2857.
Rao M, Casimiro MC, Lisanti MP, D’Amico M, Wang C, Shirley LA, et al. Inhibition of cyclin D1 gene transcription by Brg-1. Cell Cycle. 2008;7(5):647–55.
Vierbuchen T, Ling E, Cowley CJ, Couch CH, Wang X, Harmin DA, et al. AP-1 Transcription factors and the BAF complex mediate signal-dependent enhancer selection. Mol Cell. 2017;68(6):1067–1082 e12.
Kelso TWR, Porter DK, Amaral ML, Shokhirev MN, Benner C, Hargreaves DC. Chromatin accessibility underlies synthetic lethality of SWI/SNF subunits in ARID1A-mutant cancers. Elife. 2017;6:e30506.
Hastings JM, Jackson KS, Mavrogianis PA, Fazleabas AT. The estrogen early response gene FOS is altered in a baboon model of endometriosis. Biol Reprod. 2006;75(2):176–82.
Morsch DM, Carneiro MM, Lecke SB, Araujo FC, Camargos AF, Reis FM, et al. c-fos gene and protein expression in pelvic endometriosis: a local marker of estrogen action. J Mol Histol. 2009;40(1):53–8.
Boettger-Tong HL, Murthy L, Stancel GM. Cellular pattern of c-fos induction by estradiol in the immature rat uterus. Biol Reprod. 1995;53(6):1398–406.
Hyder SM, Stancel GM, Nawaz Z, McDonnell DP, Loose-Mitchell DS. Identification of an estrogen response element in the 3’-flanking region of the murine c-fos protooncogene. J Biol Chem. 1992;267(25):18047–54.
Papa M, Mezzogiorno V, Bresciani F, Weisz A. Estrogen induces c-fos expression specifically in the luminal and glandular epithelia of adult rat uterus. Biochem Biophys Res Commun. 1991;175(2):480–5.
Shemshedini L, Knauthe R, Sassone-Corsi P, Pornon A, Gronemeyer H. Cell-specific inhibitory and stimulatory effects of Fos and Jun on transcription activation by nuclear receptors. EMBO J. 1991;10(12):3839–49.
Hastings JM, Fazleabas AT. A baboon model for endometriosis: implications for fertility. Reprod Biol Endocrinol. 2006;4(Suppl 1):S7.
Pan H, Sheng JZ, Tang L, Zhu R, Zhou TH, Huang HF. Increased expression of c-fos protein associated with increased matrix metalloproteinase-9 protein expression in the endometrium of endometriotic patients. Fertil Steril. 2008;90(4):1000–7.
Salmi A, Heikkila P, Lintula S, Rutanen EM. Cellular localization of c-jun messenger ribonucleic acid and protein and their relation to the proliferation marker Ki-67 in the human endometrium. J Clin Endocrinol Metab. 1998;83(5):1788–96.
Beste MT, Pfaffle-Doyle N, Prentice EA, Morris SN, Lauffenburger DA, Isaacson KB, et al. Molecular network analysis of endometriosis reveals a role for c-Jun-regulated macrophage activation. Sci Transl Med. 2014;6(222):222ra16.
Ding S, Yu Q, Wang J, Zhu L, Li T, Guo X, et al. Activation of ATF3/AP-1 signaling pathway is required for P2X3-induced endometriosis pain. Hum Reprod. 2020;35(5):1130–44.
Zeitvogel A, Baumann R, Starzinski-Powitz A. Identification of an invasive, N-cadherin-expressing epithelial cell type in endometriosis using a new cell culture model. Am J Pathol. 2001;159(5):1839–52.
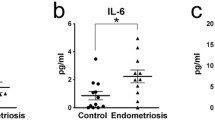
Song Y, Su RW, Joshi NR, Kim TH, Lessey BA, Jeong JW, et al. Interleukin-6 (IL-6) activates the NOTCH1 signaling pathway through e-proteins in endometriotic lesions. J Clin Endocrinol Metab. 2020;105(5):1316–1326.
Colon-Caraballo M, Torres-Reveron A, Soto-Vargas JL, Young SL, Lessey B, Mendoza A, et al. Effects of histone methyltransferase inhibition in endometriosis. Biol Reprod. 2018;99(2):293–307.
Yoo JY, Jeong JW, Fazleabas AT, Tayade C, Young SL, Lessey BA. Protein inhibitor of activated STAT3 (PIAS3) is down-regulated in eutopic endometrium of women with endometriosis. Biol Reprod. 2016;95(1):11.
Joshi NR, Su RW, Chandramouli GV, Khoo SK, Jeong JW, Young SL, et al. Altered expression of microRNA-451 in eutopic endometrium of baboons (Papio anubis) with endometriosis. Hum Reprod. 2015;30(12):2881–91.
Ruiz LA, Baez-Vega PM, Ruiz A, Peterse DP, Monteiro JB, Bracero N, et al. Dysregulation of lysyl oxidase expression in lesions and endometrium of women with endometriosis. Reprod Sci. 2015;22(12):1496–508.
Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–7.
Liberzon A, Birger C, Thorvaldsdottir H, Ghandi M, Mesirov JP, Tamayo P. The molecular signatures database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1(6):417–25.
Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics. 2016;32(18):2847–9.
Larsson J. eulerr: area-proportional euler and venn diagrams with ellipses. R package version 6.1.0. R Foundation for Statistical Computing. 2020.
Wickham, H. ggplot2: elegant graphics for data analysis. New York: Springer-Verlag; 2016.
R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2018.
Gervasi M, Bianchi-Smiraglia A, Cummings M, Zheng Q, Wang D, Liu S, et al. JunB contributes to Id2 repression and the epithelial-mesenchymal transition in response to transforming growth factor-beta. J Cell Biol. 2012;196(5):589–603.
Chang H, Liu Y, Xue M, Liu H, Du S, Zhang L, et al. Synergistic action of master transcription factors controls epithelial-to-mesenchymal transition. Nucleic Acids Res. 2016;44(6):2514–27.
Nieto MA, Huang RY, Jackson RA, Thiery JP. Emt: 2016. Cell. 2016;166(1):21–45.
Wang WM, Wu SY, Lee AY, Chiang CM. Binding site specificity and factor redundancy in activator protein-1-driven human papillomavirus chromatin-dependent transcription. J Biol Chem. 2011;286(47):40974–86.
Chen YJ, Chang LS. NFkappaB- and AP-1-mediated DNA looping regulates matrix metalloproteinase-9 transcription in TNF-alpha-treated human leukemia U937 cells. Biochim Biophys Acta. 2015;1849(10):1248–59.
Vercellini P, Vigano P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10(5):261–75.
Yamauchi N, Harada T, Taniguchi F, Yoshida S, Iwabe T, Terakawa N. Tumor necrosis factor-alpha induced the release of interleukin-6 from endometriotic stromal cells by the nuclear factor-kappaB and mitogen-activated protein kinase pathways. Fertil Steril. 2004;82(Suppl 3):1023–8.
Chatterjee K, Jana S, DasMahapatra P, Swarnakar S. EGFR-mediated matrix metalloproteinase-7 up-regulation promotes epithelial-mesenchymal transition via ERK1-AP1 axis during ovarian endometriosis progression. FASEB J. 2018;32(8):4560–72.
Acknowledgements
We thank Drs. Jose Teixeira, John Risinger, Jeff MacKeigan, Peter Laird, Jamie Bernard, Victoria Bae-Jump, and Asgi Fazleabas for helpful discussions. M.R.W. was supported by an American Cancer Society Postdoctoral Fellowship (PF-17-163-02-DDC) and a National Cancer Institute Pathway to Independence Award (K99 CA252152). R.L.C was supported by a grant from the National Institute for Child Health and Human Development (R21 HD099383).
Author information
Authors and Affiliations
Contributions
Conceptualization: M.R.W. and R.L.C. Investigation: M.R.W. Methodology: M.R.W., J.J.R., and R.L.C. Resources: R.L.C. Formal analysis: M.R.W. and J.J.R. Data curation: M.R.W. and J.J.R. Writing—original draft: M.R.W. Writing—review and editing: M.R.W., J.J.R., and R.L.C. Funding acquisition: M.R.W. and R.L.C. Supervision: R.L.C. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wilson, M.R., Reske, J.J. & Chandler, R.L. AP-1 Subunit JUNB Promotes Invasive Phenotypes in Endometriosis. Reprod. Sci. 29, 3266–3277 (2022). https://doi.org/10.1007/s43032-022-00974-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-00974-3