Abstract
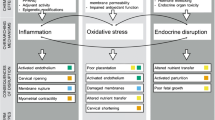
Preterm birth occurs disproportionately in the USA non-Hispanic Black population. Black women also face disproportionate exposure to certain environmental chemicals. The goal of this study was to use publicly available toxicogenomic data to identify chemical exposures that may contribute to preterm birth disparities. We tested 19 chemicals observed at higher levels in the blood or urine of non-Hispanic Black women compared to non-Hispanic White women. We obtained chemical-gene interactions from the Comparative Toxicogenomics Database and a list of genes involved in preterm birth from the Preterm Birth Database. We tested chemicals for enrichment with preterm birth genes using chi-squared tests. We then conducted pathway enrichment analysis for the preterm birth genes using DAVID software and identified chemical impacts on genes involved in these pathways. Genes annotated to all 19 chemicals were enriched with preterm birth genes (FDR-adjusted p value < 0.05). Preterm birth enriched chemicals that were detected at the highest levels in non-Hispanic Black women included methyl mercury, methylparaben, propylparaben, diethyl phthalate, dichlorodiphenyldichloroethylene, and bisphenol S. The preterm birth genes were enriched for pathways including “inflammatory response” (FDR-adjusted p value = 3 × 10–19), “aging” (FDR-adjusted p value = 4 × 10–8) and “response to estradiol” (FDR-adjusted p value = 2 × 10–4). Chemicals enriched with preterm birth genes impacted genes in all three pathways. This study adds to the body of knowledge suggesting that exposures to environmental chemicals contribute to racial disparities in preterm birth and that multiple chemicals drive these effects. These chemicals affect genes involved in biological processes relevant to preterm birth such as inflammation, aging, and estradiol pathways.





Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
The R markdown code used to conduct these analyses and generate figures is available at https://github.com/bakulskilab.
References
Hamilton, B.M., JA., Osterman, MJK., Births: provisional data for 2019. 2020, Centers for Disease Control and Prevention.
Pike KC, Lucas JS. Respiratory consequences of late preterm birth. Paediatr Respir Rev. 2015;16(3):182–8.
Behrman, R.E., Butler, A. S., Preterm birth: causes, consequences, and prevention, R.E. Behrman and A.S. Butler, Editors. 2007: Washington (DC).
Grosse, S.D., et al., Employer-sponsored plan expenditures for infants born preterm. Pediatrics, 2017. 140(4).
Ruiz D, et al. Disparities in environmental exposures to endocrine-disrupting chemicals and diabetes risk in vulnerable populations. Diabetes Care. 2018;41(1):193–205.
Nguyen VK, et al. A comprehensive analysis of racial disparities in chemical biomarker concentrations in United States women, 1999–2014. Environ Int. 2020;137:105496.
York TP, et al. The contribution of genetic and environmental factors to the duration of pregnancy. Am J Obstet Gynecol. 2014;210(5):398–405.
Norwitz, E.R., et al., Molecular regulation of parturition: The role of the decidual clock. Cold Spring Harb Perspect Med, 2015. 5(11).
Kota SK, et al. Endocrinology of parturition. Indian J Endocrinol Metab. 2013;17(1):50–9.
Romero R, et al. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11(5):317–26.
Menon R, et al. Histological evidence of oxidative stress and premature senescence in preterm premature rupture of the human fetal membranes recapitulated in vitro. Am J Pathol. 2014;184(6):1740–51.
Schmidt A, et al. Only humans have human placentas: molecular differences between mice and humans. J Reprod Immunol. 2015;108:65–71.
Faas MM, et al. Species differences in the effect of pregnancy on lymphocyte cytokine production between human and rat. J Leukoc Biol. 2005;78(4):946–53.
Keirse MJ, et al. Comparison of intrauterine prostaglandin metabolism during pregnancy in man, sheep and guinea pig. Eur J Obstet Gynecol Reprod Biol. 1978;8(4):195–203.
Grigsby PL. Animal models to study placental development and function throughout normal and dysfunctional human pregnancy. Semin Reprod Med. 2016;34(1):11–6.
Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect. 2011;119(6):878–85.
Maekawa R, et al. Evidence of exposure to chemicals and heavy metals during pregnancy in Japanese women. Reprod Med Biol. 2017;16(4):337–48.
Boyle EB, et al. Assessment of Exposure to VOCs among Pregnant Women in the National Children’s Study. Int J Environ Res Public Health. 2016;13(4):376.
Davis AP, et al. The comparative toxicogenomics database: update 2019. Nucleic Acids Res. 2019;47(D1):D948–54.
Uzun, A., et al., dbPTB: a database for preterm birth. Database (Oxford), 2012. 2012: p. bar069.
Davis AP, et al. Comparative toxicogenomics database (CTD): update 2021. Nucleic Acids Res. 2021;49(D1):D1138–43.
Harris SM, et al. Identification of environmental chemicals targeting miscarriage genes and pathways using the comparative toxicogenomics database. Environ Res. 2020;184:109259.
Campbell I. Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Stat Med. 2007;26(19):3661–75.
Saeed AI, et al. TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003;34(2):374–8.
Camargo A, et al. Permutation - based statistical tests for multiple hypotheses. Source Code Biol Med. 2008;3:15.
Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57.
Cappelletti M, et al. Inflammation and preterm birth. J Leukoc Biol. 2016;99(1):67–78.
Polettini J, et al. Aging of intrauterine tissues in spontaneous preterm birth and preterm premature rupture of the membranes: a systematic review of the literature. Placenta. 2015;36(9):969–73.
Menon R, Richardson LS, Lappas M. Fetal membrane architecture, aging and inflammation in pregnancy and parturition. Placenta. 2019;79:40–5.
Smith R. Parturition. N Engl J Med. 2007;356(3):271–83.
Martin, J.A., et al., Births: final data for 2018, in National Vital Statistics Reports. 2019.
James-Todd TM, Chiu YH, Zota AR. Racial/ethnic disparities in environmental endocrine disrupting chemicals and women’s reproductive health outcomes: epidemiological examples across the life course. Curr Epidemiol Rep. 2016;3(2):161–80.
Helm JS, et al. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by Black women. Environ Res. 2018;165:448–58.
Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA Pediatr. 2014;168(1):61–7.
Polanska K, et al. Effect of environmental phthalate exposure on pregnancy duration and birth outcomes. Int J Occup Med Environ Health. 2016;29(4):683–97.
Radke EG, et al. Phthalate exposure and female reproductive and developmental outcomes: a systematic review of the human epidemiological evidence. Environ Int. 2019;130:104580.
Baker BH, et al. Methylparaben in meconium and risk of maternal thyroid dysfunction, adverse birth outcomes, and attention-deficit hyperactivity disorder (ADHD). Environ Int. 2020;139:105716.
Zota, A.R. and B. Shamasunder, The environmental injustice of beauty: framing chemical exposures from beauty products as a health disparities concern. Am J Obstet Gynecol, 2017. 217(4): p. 418 e1-418 e6.
Bristor J, Gravois R, Hunt M. Race and ideology: African-American images in television advertising. J Public Policy Mark. 1995;14(1):48–59.
Bravo MA, et al. Racial isolation and exposure to airborne particulate matter and ozone in understudied US populations: environmental justice applications of downscaled numerical model output. Environ Int. 2016;92–93:247–55.
Morello-Frosch R, Jesdale BM. Separate and unequal: residential segregation and estimated cancer risks associated with ambient air toxics in U.S. metropolitan areas. Environ Health Perspect. 2006;114(3):386–93.
Nardone A, et al. Redlines and greenspace: The relationship between historical redlining and 2010 greenspace across the united states. Environ Health Perspect. 2021;129(1):17006.
Davis HT, et al. Potential sources and racial disparities in the residential distribution of soil arsenic and lead among pregnant women. Sci Total Environ. 2016;551–552:622–30.
Chen H, et al. Contamination features and health risk of soil heavy metals in China. Sci Total Environ. 2015;512–513:143–53.
Li, S., et al., Heavy Metal(loid)s Contamination in ground dust and associated health risks at a former indigenous zinc smelting area. Int J Environ Res Public Health, 2021. 18(3).
Hogervorst J, et al. House dust as possible route of environmental exposure to cadmium and lead in the adult general population. Environ Res. 2007;103(1):30–7.
Murphy BL, Toole AP, Bergstrom PD. Health risk assessment for arsenic contaminated soil. Environ Geochem Health. 1989;11(3–4):163–9.
Juhasz AL, Weber J, Smith E. Impact of soil particle size and bioaccessibility on children and adult lead exposure in peri-urban contaminated soils. J Hazard Mater. 2011;186(2–3):1870–9.
Almberg KS, et al. Arsenic in drinking water and adverse birth outcomes in Ohio. Environ Res. 2017;157:52–9.
Ahmad SA, et al. Arsenic in drinking water and pregnancy outcomes. Environ Health Perspect. 2001;109(6):629–31.
Laine JE, et al. Maternal arsenic exposure, arsenic methylation efficiency, and birth outcomes in the Biomarkers of Exposure to ARsenic (BEAR) pregnancy cohort in Mexico. Environ Health Perspect. 2015;123(2):186–92.
Torres-Sanchez LE, et al. Intrauterine lead exposure and preterm birth. Environ Res. 1999;81(4):297–301.
Zhang B, et al. Prenatal exposure to lead in relation to risk of preterm low birth weight: A matched case-control study in China. Reprod Toxicol. 2015;57:190–5.
Mohai P, et al. Racial and socioeconomic disparities in residential proximity to polluting industrial facilities: evidence from the Americans’ Changing Lives Study. Am J Public Health. 2009;99(Suppl 3):S649–56.
Frank JJ, et al. Systematic review and meta-analyses of lead (Pb) concentrations in environmental media (soil, dust, water, food, and air) reported in the United States from 1996 to 2016. Sci Total Environ. 2019;694:133489.
ThanosBourtsalas AC, Themelis NJ. Major sources of mercury emissions to the atmosphere: The U.S. case. Waste Manag. 2019;85:90–4.
Cheng L, et al. Fetal exposure to lead during pregnancy and the risk of preterm and early-term deliveries. Int J Hyg Environ Health. 2017;220(6):984–9.
Kim SS, et al. Urinary trace metals individually and in mixtures in association with preterm birth. Environ Int. 2018;121(Pt 1):582–90.
Aung MT, et al. Preterm birth in relation to the bisphenol A replacement, bisphenol S, and other phenols and parabens. Environ Res. 2019;169:131–8.
Geer LA, et al. Association of birth outcomes with fetal exposure to parabens, triclosan and triclocarban in an immigrant population in Brooklyn. New York J Hazard Mater. 2017;323(Pt A):177–83.
Tsukimori K, et al. Long-term effects of polychlorinated biphenyls and dioxins on pregnancy outcomes in women affected by the Yusho incident. Environ Health Perspect. 2008;116(5):626–30.
Ferguson KK, O’Neill MS, Meeker JD. Environmental contaminant exposures and preterm birth: a comprehensive review. J Toxicol Environ Health B Crit Rev. 2013;16(2):69–113.
Aker AM, et al. The associations between prenatal exposure to triclocarban, phenols and parabens with gestational age and birth weight in northern Puerto Rico. Environ Res. 2019;169:41–51.
Wan Y, et al. Relationship between maternal exposure to bisphenol S and pregnancy duration. Environ Pollut. 2018;238:717–24.
Kezios KL, et al. Dichlorodiphenyltrichloroethane (DDT), DDT metabolites and pregnancy outcomes. Reprod Toxicol. 2013;35:156–64.
Koniecki D, et al. Phthalates in cosmetic and personal care products: concentrations and possible dermal exposure. Environ Res. 2011;111(3):329–36.
Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345(6198):760–5.
Welsh T, et al. Estrogen receptor (ER) expression and function in the pregnant human myometrium: estradiol via ERalpha activates ERK1/2 signaling in term myometrium. J Endocrinol. 2012;212(2):227–38.
Mercer, B.M., Preterm premature rupture of the membranes: diagnosis and management. Clin Perinatol, 2004. 31(4): p. 765-82, vi.
Hashimoto Y, et al. Measurement of estrogenic activity of chemicals for the development of new dental polymers. Toxicol In Vitro. 2001;15(4–5):421–5.
Kang JS, et al. Estrogenic potency of bisphenol S, polyethersulfone and their metabolites generated by the rat liver S9 fractions on a MVLN cell using a luciferase reporter gene assay. Reprod Biol Endocrinol. 2014;12:102.
Wood SL, et al. Endocrine disruptors and spontaneous premature labor: a case control study. Environ Health. 2007;6:35.
Funding
This research was supported by the Michigan Center on Lifestage Environmental Exposures and Disease (P30ES017885). Drs. Harris, Bakulski and Loch-Caruso were supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number P42ES017198. Additional funding for Dr. Harris was provided by the National Center for Advancing Translational Sciences (UL1TR002240). Dr. Bakulski was supported by grants from the National Institutes of Health (R01ES025531; R01ES025574; R01AG055406; R01MD013299). Dr. Colacino and Dr. Nguyen were supported by grants from the National Institutes of Health (R01ES028802 and R01ES028802S1). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Harris, S.M., Colacino, J., Buxton, M. et al. A Data Mining Approach Reveals Chemicals Detected at Higher Levels in Non-Hispanic Black Women Target Preterm Birth Genes and Pathways. Reprod. Sci. 29, 2001–2012 (2022). https://doi.org/10.1007/s43032-022-00870-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-00870-w