Abstract
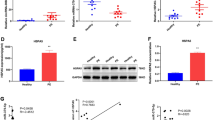
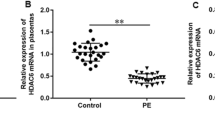
Heat shock protein alpha 8 (HSPA8) was found to be downregulated in the placentas of patients with hypertensive disorders in pregnancy (HDP). We aim to explore the underlying role and mechanism of HSPA8 in HDP progression. Herein, HSPA8 mRNA expression in placentas and peripheral blood of patients with HDP and normal pregnant controls was measured with RT-qPCR. We found that HSPA8 expression was downregulated in placentas and peripheral blood of patients with HDP. HTR8/SVneo human trophoblast cells were transfected with pcDNA-HSPA8 or si-HSPA8. HSPA8 overexpression promoted cell proliferation, migration, and MMP-2 and MMP-9 protein levels, and inhibited apoptosis, while HSPA8 silencing showed the opposite results. Co-immunoprecipitation assay validated the binding between HSPA8 and β-arrestin1, as well as β-arrestin1 and A1AR proteins. HSPA8 bound with β-arrestin1 protein and promoted β-arrestin1 expression. β-arrestin1 bound with A1AR protein and inhibited A1AR expression. Then, HTR8/SVneo cells were transfected with pcDNA-HSPA8 alone or together with si-β-arrestin1, as well as transfected with pcDNA-β-arrestin1 alone or together with pcDNA-A1AR. β-arrestin1 silencing reversed the effects of HSPA8 overexpression on HTR8/SVneo cell functions. β-arrestin1 overexpression promoted cell proliferation migration, and MMP-2 and MMP-9 protein levels, and inhibited apoptosis, while these effects were reversed by A1AR overexpression. Lentivirus HSPA8 overexpression vector (Lv-HSPA8) was injected into a preeclampsia (PE) rat model, which attenuated blood pressure and fetal detrimental changes in PE rats. In conclusion, HSPA8 promoted proliferation and migration and inhibited apoptosis in trophoblast cells, and attenuated the symptoms of PE rats by modulating the β-arrestin1/A1AR axis. Our study provided a novel theoretical evidence and potential strategy for HDP treatment.







Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Tranquilli AL, Dekker G, Magee L, Roberts J, Sibai BM, Steyn W, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens. 2014;4(2):97–104.
Chen DB, Wang W. Human placental microRNAs and preeclampsia. Biol Reprod. 2013;88(5):130.
Farrell A, Alahari S, Ermini L, Tagliaferro A, Litvack M, Post M, et al. Faulty oxygen sensing disrupts angiomotin function in trophoblast cell migration and predisposes to preeclampsia. JCI Insight. 2019;4(8):e127009.
Wu L, Zhao KQ, Wang W, Cui LN, Hu LL, Jiang XX, et al. Nuclear receptor coactivator 6 promotes HTR-8/SVneo cell invasion and migration by activating NF-κB-mediated MMP9 transcription. Cell Prolif. 2020;53(9):e12876.
Liu T, Daniels CK, Cao S. Comprehensive review on the HSC70 functions, interactions with related molecules and involvement in clinical diseases and therapeutic potential. Pharmacol Ther. 2012;136(3):354–74.
Grifoni SC, McKey SE, Drummond HA. Hsc70 regulates cell surface ASIC2 expression and vascular smooth muscle cell migration. Am J Physiol Heart Circ Physiol. 2008;294(5):H2022–30.
Gharesi-Fard B, Zolghadri J, Kamali-Sarvestani E. Proteome differences of placenta between pre-eclampsia and normal pregnancy. Placenta. 2010;31(2):121–5.
Tong C, Feng X, Chen J, Qi X, Zhou L, Shi S, et al. G protein-coupled receptor 30 regulates trophoblast invasion and its deficiency is associated with preeclampsia. J Hypertens. 2016;34(4):710–8.
Motawea HKB, Chotani MA, Ali M, Ackerman W, Zhao G, Ahmed AAE, et al. Human Placenta Expresses α(2)-Adrenergic Receptors and May Be Implicated in Pathogenesis of Preeclampsia and Fetal Growth Restriction. Am J Pathol. 2018;188(12):2774–85.
Borea PA, Gessi S, Merighi S, Varani K. Adenosine as a Multi-Signalling Guardian Angel in Human Diseases: When, Where and How Does it Exert its Protective Effects? Trends Pharmacol Sci. 2016;37(6):419–34.
Brown RD, Thorén P, Steege A, Mrowka R, Sällström J, Skøtt O, et al. Influence of the adenosine A1 receptor on blood pressure regulation and renin release. Am J Physiol Regul Integr Comp Physiol. 2006;290(5):R1324–9.
von Versen-Höynck F, Rajakumar A, Bainbridge SA, Gallaher MJ, Roberts JM, Powers RW. Human placental adenosine receptor expression is elevated in preeclampsia and hypoxia increases expression of the A2A receptor. Placenta. 2009;30(5):434–42.
Salsoso R, Farías M, Gutiérrez J, Pardo F, Chiarello DI, Toledo F, et al. Adenosine and preeclampsia. Mol Aspects Med. 2017;55:126–39.
Jean-Charles PY, Kaur S, Shenoy SK. G Protein-Coupled Receptor Signaling Through β-Arrestin-Dependent Mechanisms. J Cardiovasc Pharmacol. 2017;70(3):142–58.
Jajoo S, Mukherjea D, Kumar S, Sheth S, Kaur T, Rybak LP, et al. Role of beta-arrestin1/ERK MAP kinase pathway in regulating adenosine A1 receptor desensitization and recovery. Am J Physiol Cell Physiol. 2010;298(1):C56-65.
Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018;13:291–310.
Kemse NG, Kale AA, Joshi SR. Supplementation of maternal omega-3 fatty acids to pregnancy induced hypertension Wistar rats improves IL10 and VEGF levels. Prostaglandins Leukot Essent Fatty Acids. 2016;104:25–32.
Luo JY, Fu D, Wu YQ, Gao Y. Inhibition of the JAK2/STAT3/SOSC1 Signaling Pathway Improves Secretion Function of Vascular Endothelial Cells in a Rat Model of Pregnancy-Induced Hypertension. Cell Physiol Biochem. 2016;40(3–4):527–37.
Tsutsui S, Vergote D, Shariat N, Warren K, Ferguson SS, Power C. Glucocorticoids regulate innate immunity in a model of multiple sclerosis: reciprocal interactions between the A1 adenosine receptor and beta-arrestin-1 in monocytoid cells. Faseb j. 2008;22(3):786–96.
Wang N, Feng Y, Xu J, Zou J, Chen M, He Y, et al. miR-362-3p regulates cell proliferation, migration and invasion of trophoblastic cells under hypoxia through targeting Pax3. Biomed Pharmacother. 2018;99:462–8.
Duan B, Zhang L, Ding X, Li L, Li Y, Geng H, et al. Serum Beta-Trace Protein as a Novel Predictor of Pregnancy-Induced Hypertension. J Clin Hypertens (Greenwich). 2016;18(10):1022–6.
Sadeh-Mestechkin D, Epstein Shochet G, Pomeranz M, Fishman A, Drucker L, Biron-Shental T, et al. The effect of heat shock protein 27 on extravillous trophoblast differentiation and on eukaryotic translation initiation factor 4E expression. Mol Hum Reprod. 2014;20(5):422–32.
Li F, Xie Y, Wu Y, He M, Yang M, Fan Y, et al. HSP20 Exerts a Protective Effect on Preeclampsia by Regulating Function of Trophoblast Cells Via Akt Pathways. Reprod Sci. 2019;26(7):961–71.
Luttrell LM, Gesty-Palmer D. Beyond desensitization: physiological relevance of arrestin-dependent signaling. Pharmacol Rev. 2010;62(2):305–30.
Quitterer U, Fu X, Pohl A, Bayoumy KM, Langer A, AbdAlla S. Beta-Arrestin1 Prevents Preeclampsia by Downregulation of Mechanosensitive AT1-B2 Receptor Heteromers. Cell. 2019;176(1–2):318-333.e19.
Sun JC, Liu B, Zhang RW, Jiao PL, Tan X, Wang YK, et al. Overexpression of ß-Arrestin1 in the Rostral Ventrolateral Medulla Downregulates Angiotensin Receptor and Lowers Blood Pressure in Hypertension. Front Physiol. 2018;9:297.
Iriyama T, Sun K, Parchim NF, Li J, Zhao C, Song A, et al. Elevated Placental Adenosine Signaling Contributes to the Pathogenesis of Preeclampsia. Circulation. 2015;131(8):730–41.
Gao X, Patzak A, Sendeski M, Scheffer PG, Teerlink T, Sällström J, et al. Adenosine A1-receptor deficiency diminishes afferent arteriolar and blood pressure responses during nitric oxide inhibition and angiotensin II treatment. Am J Physiol Regul Integr Comp Physiol. 2011;301(6):R1669–81.
Darashchonak N, Koepsell B, Bogdanova N, von Versen-Höynck F. Adenosine A2B receptors induce proliferation, invasion and activation of cAMP response element binding protein (CREB) in trophoblast cells. BMC Pregnancy Childbirth. 2014;14:2.
Author information
Authors and Affiliations
Contributions
KZ designed the experiments. KZ, HZ, FW, and SG performed the experimental work. CS provided statistical analysis as well as figures and table for the manuscript. KZ wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This study was approved by the Ethnic Committee of the Second Affiliated Hospital of Zhengzhou University. All animal care and experimental procedures in this study were approved by Animal Care and Use Committee of the Second Affiliated Hospital of Zhengzhou University.
Consent to Participate
This study was approved by the Ethnic Committee of the Second Affiliated Hospital of Zhengzhou University, and all subjects had read and signed the informed consent.
Consent for Publication
All authors have written the informed consent for publication.
Conflict of Interest
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Zhang, K., Zhang, H., Wang, F. et al. HSPA8 Is Identified as a Novel Regulator of Hypertensive Disorders in Pregnancy by Modulating the β-Arrestin1/A1AR Axis. Reprod. Sci. 29, 564–577 (2022). https://doi.org/10.1007/s43032-021-00719-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00719-8