Abstract
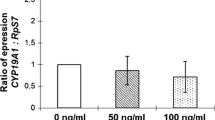
Anti-Müllerian hormone (AMH) downregulates the level of stem cell factor (SCF) via the cAMP/PKA signaling pathway in human granulosa cells (GCs). Little information is available on the molecular mechanism underlying the interaction. This study is aimed at determining whether AMH regulates expression of SCF via the cAMP-PKA-CREB signaling pathway in human GCs. In the present study, we verified the binding of cAMP-response element-binding protein (CREB) to promoter of SCF in human GCs. Furthermore, the effect of CREB was tested on the SCF promoter, and the site of CREB binding to SCF promoter was identified using truncations as well as assays of SCF-promoted mutation and CREB mutation. To investigate the correlation among AMH, SCF promoter, and CREB, pGL-Basic-SCF+CREB was transfected into overexpressed AMH GCs (AMH-high GCs), low expressed AMH GCs (AMH-low GCs), and normal GCs (GCs), respectively. Finally, immunofluorescence, double immunostaining, and Western blot were carried out in AMH-high and AMH-low GCs to confirm the AMH-mediated regulation of SCF expression by inhibiting the phosphorylation of CREB (pCREB) in GCs. Results indicated CREB interacted with SCF promoter and significantly enhanced the transcription level of SCF. The CREB binding site was localized at 318–321 bp of SCF gene promote. AMH inhibits the expression of SCF by phosphorylation of CREB via the PKA signaling pathway in GCs. These findings provide an in-depth understanding of the molecular mechanism underlying AMH suppressing the follicle growth, which would aid in the development of a novel therapy.





Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Cate RL, Mattaliano RJ, Hession C. Isolation of the bovine and human genes for Mullerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986;45(5):685–9834.
Blanchard MG, Josso N. Source of the anti-Müllerian hormone synthesized by the fetal testis: Müllerian-inhibiting activity of fetal bovine Sertoli cells in tissue culture. Pediatr Res. 1974;8(12):968–71.
Modi D, Bhartiya D, Puri C. Developmental expression and cellular distribution of Mullerian inhibiting substance in the primate ovary. Reproduction. 2006;132(3):443–53.
Be’zard J, Vigier B, Tran D. Immunocytochemical study of anti-Mu¨llerian hormone in sheep ovarian follicles during fetal and postnatal development. J Reprod Fertil. 1987;80:509–16.
Weenen C, Laven JS, Von Bergh AR. Antimullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10(2):77–83.
Fiçicioglu C, Kutlu T, Baglam E, Bakacak Z. Early follicular antimullerianhormone as an indicator of ovarian reserve. Fertil Steril. 2006;85:592–6.
La Marca A, Giulini S, Tirelli A, Bertucci E, Marsella T, Xella S, et al. Antimullerian hormone measurement on any day of the menstrual cycle strongly predicts ovarian response in assisted reproductive technology. Hum Reprod. 2007;22:766–71.
Nilsson E, Rogers N, Skinner MK. Actions of antimullerian hormone on the ovarian transcriptome to inhibit primordial to primary follicle transition. Reproduction. 2007;134:209–21.
Sowers MR, Eyvazzadeh AD, McConnell D. Antimullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab. 2008;93:3478–83.
Lie Fong S, Baart EB, Martini E. Anti-Mu ¨llerian hormone: a marker for oocyte quantity, oocyte quality and embryo quality? Reprod BioMed Online. 2008;16:664–70.
Reddy P, Shen L, Ren C, et al. Activation of Akt (PKB) and suppression of FKHRL1 in mouse and rat oocytes by stem cell factor during follicular activation and development. Dev Biol. 2005;281:160–70.
McLaughlin EA, McIver SC. Awakening theoocyte: controlling primordial follicle development. Reproduction. 2009;137:1–11.
Hu R, Wang FM, Yu L, et al. Antimullerian hormone regulates stem cell factor expression in human granulosa cells. Fertil Steril. 2014;102:1742–50 e1741.
Kanakkaparambil R, Singh R, Li D, Webb R, Sinclair KD. B-vitamin and homocysteine status determines ovarian response to gonadotropin treatment in sheep. Biol Reprod. 2009;80:743–52.
Gruijters MJG, Visser JA, Durlinger ALL, Themmen AP. Antimullerian hormone and its role in ovarian function. Mol Cell Endocrinol. 2003;211:85–90.
Liu B, Zhang L, Guo RW, Wang WJ, Duan XQ, Liu YW. The serum level of C-reactive protein in patients undergoing GnRH agonist protocols for in vitro fertilization cycle. Clin Exp Obstet Gynecol. 2014;41:190–4.
Yalcinkaya E, Cakiroglu Y, Do ger E, et al. Effect of follicular fluid NO, MDA and GSH levels on in vitro fertilization outcomes. J Turk Ger Gynecol Assoc. 2013;14:136–41.
Hu YW, Zhao JY, Li SF. RP5833A20.1/miR-382-5p/NFIA-dependent signal transduction pathway contributes to the regulation of cholesterol homeostasis and inflammatory reaction. Arterioscler Thromb Vasc Biol. 2015;35:87–101.
Rapicavoli NA, Qu K, Zhang J, et al. A mammalian pseudogene lncRNA at the interface of inflammation and anti-inflammatory therapeutics. Elife. 2013;2:e00762.
Hu R, Lou Y, Wang F-M. Effects of recombinant human AMH on SCF expression in human granulosa cells. Cell Biochem Biophys. 2013;67(3):1481–5.
Macklon NS, Fauser BC. Ovarian reserve. Semin Reprod Med. 2005;23:248–56.
Visser JA. AMH signaling: from receptor to target gene. Mol Cell Endocrinol. 2003;211:65–7.
Hirobe S, He WW, Gustafson ML. Müllerian inhibiting substance gene expression in the cycling rat ovary correlates with recruited or graafian follicle selection. Biol Reprod. 1994;50(6):1238–43.
Lassot I, Ségéral E, Berlioz-Torrent C. ATF4 degradation relies on a phosphorylation-dependent interaction with the SCF ubiquitin ligase. Mol Cell Biol. 2001;21(6):2192–202.
Acknowledgments
We thank the Laboratory of Fertility Maintenance of Ningxia Medical University and the Clinical Pathogen Microbiology Laboratory of the General Hospital of Ningxia Medical University for providing an experimental platform. And we thank professor Hai Wang for modifying the article.
Funding
This work was supported by the Natural Science Foundation of China (No. 81660257), Ningxia Natural Science Foundation (NZ16125, NZ16143), Personnel Agency Overseas talent Project, 2017 Autonomous Region Leader in Science and Technology Innovation, First-Class Discipline Construction Founded Project of Ningxia Medical University and the School of Clinical Medicine (NXYLXK2017A05), and 2018 Advantageous Subjects Project of Ningxia Medical University (XY201807). Key Research and Development program of Ningxia Hui Autonomous Region (2019BFG02005).
Author information
Authors and Affiliations
Contributions
YXF and FMW conceived and designed the study with inputs from RH. YXF and FMW have the same contribution to this article. FMW and RH were responsible for the supervision and coordination of the project. YXF and XE performed most of the in vitro experiments. HMY collected the FF. TH and HW led the data analysis with inputs from YXF, Yanfei-Wang, XE, Yafei-Wang, and HMY. The first draft of the manuscript was written by YXF and FMW; then, RH, XE, Yafei-Wang, and Yanfei-Wang contributed to revise and review the manuscript. All authors read and approved the final manuscript before submission.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The use of human follicular fluid was reviewed and approved by the Ethics Committee of Ningxia Medical University and was performed in accordance with the approved guidelines. Written informed consent was obtained from each participating patient.
Competing Interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fu, YX., Wang, FM., Ou-yang, XE. et al. Anti-Müllerian Hormone Regulates Stem Cell Factor via cAMP/PKA Signaling Pathway in Human Granulosa Cells by Inhibiting the Phosphorylation of CREB. Reprod. Sci. 27, 325–333 (2020). https://doi.org/10.1007/s43032-019-00033-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-019-00033-4