Abstract
Myosorex varius is a South African generalist shrew, which has been found to consist of several genetic clades across its range. A northern clade inhabits the more mesic, summer-rainfall areas of grassland and savannah in the east of South Africa. A southern clade occupies areas of fynbos in the south, and can be further divided into a western subclade occupying winter-rainfall areas and an eastern subclade occupying areas with aseasonal rainfall. Non-South-African members of the African genus Myosorex primarily are limited to isolated montane habitats along the East African Rift System. Here, we used palaeoclimatic niche modelling to examine the effects of Pliocene and Pleistocene climate change on the distributions of M. varius, its clades and the genus as a whole. Results indicate that repeated cycles of range expansion during glacial periods and fragmentation during interglacials are responsible for current phylogeographic patterns within M. varius. Based on their close alignment with rainfall zones and lack of genetic mixing despite areas of contact, it is likely that these (sub)clades are locally adapted to their respective areas. Earlier climatic fluctuations allowed the genus to ‘island hop’ south from East Africa along the East African Rift System, expanding in range during cooler periods and retreating to montane refugia during warmer periods. Areas currently occupied by Myosorex species largely correspond with predicted montane refugia that have allowed them to survive previous warm periods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environmental niche modelling involves the use of artificial intelligence algorithms to model the environmental niche of a species based on distribution data and a set of environmental variables. This niche (essentially a mathematical relationship between environmental parameters and the suitability of habitat) can then be projected using new environmental layers to predict the range of a species under new environmental conditions (Peterson 2001). Niche modelling based on climatic variables is commonly used to predict the likely effects of anthropogenic climate change on species distributions (Meynard et al. 2017; Ramírez-Preciado et al. 2019). However, climatic niches can also be projected using palaeoclimate data to predict species distributions under past climatic conditions, allowing researchers to explore the ancient range changes, colonisation routes and refugia that have produced present species distributions (Waltari and Guralnick 2009; Levinsky et al. 2013). Where phylogeographic data for a species are available, niche models can account for within-species diversity by modelling genetic clades individually (Pahad et al. 2020). Palaeoclimatic niche modelling also can be used to test phylogeographic theories regarding climatically induced range changes and lineage divergence by identifying periods of vicariance and connectivity, range expansion and contraction, and the location of refugia (Waltari et al. 2007; Pahad et al. 2020).
Myosorex is a sub-Saharan genus of shrew commonly known as forest or mouse shrews. Together with Congosorex and Surdisorex, they make up the subfamily Myosoricinae within the family Soricidae (Quérouil et al. 2001). The book ‘Mammals of Africa Vol IV: Hedgehogs, Shrews and Bats’ (Happold and Happold 2013) lists 14 species of Myosorex. However, the IUCN red list (IUCN 2022) currently lists 19 species of Myosorex, though little is known about most of them and many are recent discoveries (Kerbis Peterhans et al. 2008, 2010; Taylor et al. 2013).
Myosorex varius occupies the more mesic southern and eastern areas of South Africa. Despite its common name (forest shrew), M. varius primarily inhabits areas of savannah, fynbos and grassland. These shrews also occupy much of the Drakensburg mountain range, and have been found on rocky slopes at altitudes over 2200 m. Whilst primarily nocturnal, they can shift to more diurnal habits to avoid foraging on cold winter nights (Skinner and Chimimba 2005). They can eat a wide variety of invertebrates and will even feed from rodent carcasses (Goulden and Meester 1978) but have a high metabolic rate and will die quickly if deprived of food or water (Skinner and Chimimba 2005). This species was considered to be a wide-ranging generalist but phylogenetic studies confirmed the presence of well-differentiated genetic clades. There is a northern clade occupying summer-rainfall areas of savannah and grassland in the east of South Africa, and a southern clade primarily occupying winter and aseasonal rainfall areas of Fynbos along the south coast. The southern clade further subdivides into an eastern subclade occupying aseasonal rainfall areas and a western subclade occupying winter-rainfall areas (Willows-Munro and Matthee 2011). Members of the northern clade typically inhabit areas of dense vegetation near water (dense grass/thicket/forest) whereas those of the southern clade can be found in more arid areas with cover provided by succulent bushes (Skinner and Chimimba 2005). The breeding season relies on rainfall, with the northern clade breeding in summer whilst the southern clade, at least in the Western Cape, has been found to breed mostly in the winter months (Skinner and Chimimba 2005).
During the early Miocene (23–16 Ma), South Africa still was most mostly forested, but over the middle and late Miocene (16–5.3 Ma), global cooling led to a cooling and drying of southern Africa (Neumann and Bamford 2015). By the end of the Miocene (5.3 Ma), more modern biomes were forming, with a winter-rainfall zone in the south-western Cape leading to the emergence of fynbos and succulent vegetation (Verboom et al. 2009), and the arrival of arid adapted C4 grasses transforming large areas of woodland into grassland and savannah (Edwards et al. 2010). Further global cooling during the late Pliocene (3.2–2.6 Ma) led to the glacial cycles of the Pleistocene (from 2.6 Ma; Sosdian and Rosenthal 2009). By this time, the present-day biomes of Southern African had been established (Neumann and Bamford 2015) though glacial cycling would result in a constant shifting of the boundaries between these biomes (Partridge et al. 1999).
Based on fossil evidence, the genus Myosorex first appeared in North Africa at least 12 Ma and had reached the Cape by 5 Ma (Furió et al. 2007). Although formerly widespread on the continent, Myosorex exists today mostly as isolated relict populations (Furió et al. 2007). In the African tropics, the genus consists of multiple species each of which is limited to small patches of montane forest (Taylor et al. 2013). In southern Africa, M. varius has been able to adapt to more arid areas including savannah, grassland and fynbos whilst other Myosorex species are limited to patches of forest and riverine thicket (Willows-Munro and Matthee 2009).
Of the five species of Myosorex found in South Africa, M. varius, M. cafer, M. tenuis, and M. sclateri form a clade that diverged from M. meesteri (Zimbabwe and Mozambique) about 5.1 Ma and split to form the current species group between 4 and 2 Ma (Taylor et al. 2013). These southern African species split from the Tanzanian M. zinki around 8.9 Ma (Taylor et al. 2013) suggesting a migration route south along the mountains of the East African Rift System. The remaining South African species, M. longicaudatus, split from the Tanzanian species M. geata and M. kihaulei around 10 Ma, and is thought to be the remnant of a previous, similar southward expansion (Taylor et al. 2013; Willows-Munro and Matthee 2009). Myosorex varius itself diverged from M. tenuis (Taylor et al. 2013) which is currently found in Limpopo province, in the far north of the range of M. varius. These two species split around 2.7 Ma (around the start of Pleistocene glacial cycling), and the clades and subclades within M. varius formed some time later, around 2 Ma (Taylor et al. 2013; Willows-Munro and Matthee 2011). Since their formation, these (sub)clades have experienced little to no gene flow, despite contact between them (Willows-Munro and Matthee 2011).
The primary aim of the present study was to use paleoclimatic niche modelling to understand how Pleistocene glacial cycles led to the present distribution of Myosorex varius and its clades, and how ecologically divergent the clades have become. A secondary aim was to extend this analysis into the Pliocene, using the limited location data available for Myosorex as a whole, to see how climatic fluctuations during this period may have facilitated the spread of the genus south along the East African Rift System, leading to the arrival of M. varius in South Africa.
Methods
Data sources
Environmental data
Environmental data were downloaded from the PaleoClim online database (Brown et al. 2018). The paleoclimatic simulations used were: current (Karger et al. 2017), last glacial maximum (Karger et al. 2017), last interglacial (Otto-Bliesner et al. 2006), Pleistocene MIS19 interglacial (Brown et al. 2018), mid-Pliocene warm period (Hill 2015) and Pliocene M2 glaciation (Dolan et al. 2015). The simulations of the last glacial maximum (21 ka), the last interglacial (120 ka) and the Pleistocene MIS19 interglacial (787 ka) were used as an indication of the climatic extremes during the glacial cycles of Pleistocene (2.6 Ma to 11.7 ka), when the current clades within M. varius were forming. The simulations of the mid-Pliocene warm period (3.2 Ma) and the Pliocene M2 glaciation event (3.3 Ma) were used as an indication of the climatic extremes of the Pliocene (5.3–2.6 Ma) during the latest southward expansion of Myosorex into South Africa. The model for the current time period is based on climatic data from 1979 to 2013 and is recommended on the PaleoClim database (Brown et al. 2018) for use with the paleoclimatic simulations.
Of the 19 bioclimatic variables available, 8 were chosen to provide an ecologically relevant and understandable overview of temperature and rainfall patterns, whilst keeping the models relatively simple for a robust projection to paleoclimatic surfaces. These were: bio 1—annual mean temperature, bio 10—mean summer temperature (based on warmest quarter), bio 11—mean winter temperature (based on coldest quarter), bio 12—annual rainfall, bio 16—wet season rainfall (based on wettest quarter), bio 17—dry season rainfall (based on driest quarter), bio 18—summer rainfall (based on warmest quarter) and bio 19—winter rainfall (based on coldest quarter). For the eastern and western subclade, bio 15—precipitation seasonality was included, as this variable is thought to be a possible contributor to the divergence between the two subclades (Willows-Munro and Matthee 2011).
Location data
For M. varius, the location data were obtained from the study by Willows-Munro and Matthee (2011). This provided 57 location points spread across the range of the species, with all locations genetically sampled to confirm the species and identify the (sub)clade. The data included 36 locations for the northern clade and 23 for the southern clade (with the 2 clades co-occurring at 2 locations). Of the 23 southern clade locations, 11 were from the eastern subclade and 13 were from the western subclade (with the 2 subclades co-occurring at 1 location). Present day location data for the genus Myosorex were obtained from GBIF.org (2021). To achieve this, all the location data for Myosorex were downloaded, including samples not allocated a species but excluding fossil samples. This resulted in 681 locations spread along the mountains of the East African Rift System and in the more mesic areas of South Africa. The low number of samples for many individual species and the high number of samples only identified to genus level made it impossible to reliably model other species individually. However, these data were sufficient to attempt to model the niche of the genus as a whole, which shows a preference for mesic, seasonal and relatively cool habitat across all species. A single location point in Sri Lanka was omitted because the genus is not found outside Africa. West African species of Myosorex were represented by a single location point only, presumably due to low sampling effort in this area. To avoid the incorrect assumption that there is virtually no suitable habitat in West Africa, this single location point was left out and the niche model for Myosorex was trained on a map limited to eastern and southern Africa, which is the area of interest for our investigation into the southward dispersal of the genus.
Data processing
Environmental data layers were trimmed to the required extent and converted to ASC files in ArcGIS (ESRI 2017). Location data were converted to a CSV format.
The niche models and projections were produced using MaxEnt (Phillips et al. 2006). This programme uses maximum-entropy-based machine learning to find the simplest possible relationship between species presence and environmental data that still conforms to the location data provided (Phillips et al. 2004) and has consistently been found to perform well compared to other niche modelling algorithms (Ashraf et al. 2017; Elith et al. 2006; Heikkinen et al. 2012; Tognelli et al. 2009). It also works well with limited location data (Phillips et al. 2006).
Niche models were run using a tenfold cross-validation technique in which the location data are randomly divided into ten equal groups and the model run ten times. On each run, a different 10% of the location data are left out of model construction and used for model evaluation. The results produced are averages over the ten runs. To avoid overfitting (the construction of niche models using unrealistically complex relationships between presence and environmental variables; Bell and Schlaepfer 2016), the regularisation value in MaxEnt was raised to three. This prevents overfitting by increasing the penalisation of more complex solutions over simpler ones (Li and Wang 2013). Otherwise, the default settings were used.
The niches of M. varius and its (sub)clades were modelled and projected using the Pleistocene climate data to see how glacial cycling facilitated the formation of the current distribution of the species and its (sub)clades. The niche of Myosorex as a whole was modelled and projected using the Pliocene climate data to see how climatic fluctuations during this period facilitated the spread of the genus along the East African Rift System. The niche of the northern clade of M. varius also was projected in this way as a proxy for an initial, summer-rainfall-adapted M. varius population that had yet to expand to occupy the south coast.
Results
Niche models for Myosorex varius and its (sub)clades
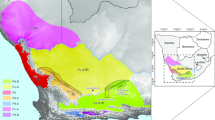
The models for M. varius and its (sub)clades performed well, with all AUC values above 0.8 (Table 1). The models for the southern clade and the subclades had higher AUC values that those for the whole species and northern clades, though this could be due to the larger amount of unoccupied territory for these maps. The projections for the present also produced range maps that closely fit the empirical presence data (Fig. 1) as well as the known range of the species (Taylor et al. 2016). Note that in the projection for the whole species (Fig. 1b), some of the location points (especially in the northern part of the northern clade) are in marginally suitable habitat only. The fit between the projected niche and the location data improved when the northern clade was modelled separately (Fig. 1c). Modelling the southern clade separately (Fig. 1d) also results in a small improvement in fit along the west coast, and modelling the eastern subclade separately (Fig. 1e) resulted in a better fit compared to models for the southern clade or the whole species. This is suggestive of local adaptation amongst the clades.
Heat maps showing the present suitability of habitat for each clade of Myosorex varius based on the niches modelled by MaxEnt. Hotter colours indicate more suitable habitat. The suitable habitat should match the location data used in model construction (shown as squares). a Map of Africa indicating the geographic location of maps. b Whole species, c northern clade, d southern clade, e western subclade and f eastern subclade
In addition to range maps, MaxEnt calculates the percentage contribution of each climatic variable to the model and produces response curves that show the relationship between a climatic variable and habitat suitability for a species or taxon being studied. Response curves have been included in an appendix. For the whole species, the most important variables were winter rainfall (74%), mean summer temperature (14%) and annual rainfall (6%). Response curves (Appendix) indicate that the species can survive in a wide range of conditions but is intolerant of extreme heat and aridity, explaining its absence from South Africa’s arid western interior and the more tropical areas to the north.
For the northern clade, the most important variables by percentage contribution were: dry season rainfall (55%), annual rainfall (24%) and mean summer temperature (17%). Summer rainfall only contributed 2% but had a permutation importance of 5%, indicating that changing this variable had an impact on model performance. Response curves (Appendix) indicate a preference for cooler, more mesic areas, similar to the species as a whole but with more emphasis on summer rainfall. The northern clade can tolerate a fairly arid dry season, but requires a wetter summer. The northern clade has suitable habitat extending into the range of the eastern subclade (Fig. 1), suggesting that it could occupy this area if it was not already occupied by the eastern subclade. An initial species adapted to the summer-rainfall zone currently occupied by the northern clade would, therefore, have already been capable of colonising part of the south coast, separated from the rest of the population by an area of less-suitable habitat (Fig. 1c).
For the southern clade, the most important variables were winter rainfall (85%) and summer rainfall (12%). Response curves (Appendix) indicate that the clade requires medium to high winter rainfall and low summer rainfall, restricting it to the winter and aseasonal rainfall zone.
For the western subclade, the most important variables were also winter rainfall (66%) and summer rainfall (31%). Response curves (Appendix) reveal environmental requirements that are similar to the southern clade as a whole but with a greater need for winter rainfall and a reduced tolerance for summer rainfall. This subclade is found in the winter-rainfall zone in the far south west of the country; however, there is a single location point within the range of the eastern subclade (Fig. 1e).
For the eastern subclade, the model was based entirely on the seasonality of rainfall (100%). The response curve (Appendix) indicates that this subclade is limited to areas of aseasonal rainfall, i.e. where rainfall is distributed evenly around the year so there is no wet/dry season. The close association of the eastern subclade with the aseasonal rainfall zone suggests that the subclade is locally adapted, as a genetically distinct population formed by vicariance alone would have to have aligned with the aseasonal rainfall zone by coincidence.
Pleistocene projections for Myosorex varius and its (sub)clades
The Pleistocene paleoprojections for M. varius and its (sub)clades (Fig. 2) indicate that ranges expanded during glacial periods and contracted during interglacials. The projections for the last interglacial are similar to those for the present day but with slightly more suitable habitat and greater range connectivity. The more ancient MIS19 interglacial projections, however, show a more severe impact on the ranges of the species and its (sub)clades, resulting in range fragmentation.
Heat maps showing the niches of Myosorex varius and its (sub)clades during Pleistocene glacial cycling. Hotter colours indicate more suitable habitat. From left to right: present, last glacial maximum (21 ka), last interglacial (130 ka) and MIS19 interglacial (787 ka). The current know range of M. tenuis has been circled in red in the present-day distribution map for the whole species (map a)
The projections for the whole species (Fig. 2a-d) indicate that, despite range connectivity being maintained during the last interglacial (Fig. 2c), the MIS19 interglacial (Fig. 2d) would have caused the northern part of the range to fragment and separate from the habitat along the south coast. The range along the south coast remained relatively continuous, although this whole species projection ignores any local adaptation. It is also worth noting the small areas of suitable habitat in the far north of the range (circled in red in Fig. 2a) which also became isolated during the MIS19 interglacial. This is the area currently occupied by M. tenuis, the sister species to M. varius. The two are hypothesised to have diverged approximately 2.7 Ma, around the start of the Pleistocene glacial cycles, and the range of M. tenuis currently is within that of M. varius (Taylor et al. 2013). Unfortunately, there is insufficient location data available currently for M. tenuis to adequately model its niche. However, the niche projections for M. varius suggest that an early interglacial similar to the MIS19 interglacial was responsible for the divergence between the two species, and that the current range overlap is due to the expansion of M. varius after reproductive isolation was established.
The projections for the northern clade (Fig. 2e–h) follow the pattern of range connectivity increasing during the glacial period and decreasing during interglacials, with the MIS19 interglacial (Fig. 2h) resulting in severe fragmentation. Here we see the northern clade fragment to form a central cluster of refugia that is well separated from any suitable habitat on the south coast. These projections suggest that an initial species with the habitat requirements of the northern clade would have been able to colonise the south coast during glacial periods, but would have been isolated from south coast populations during interglacials. The isolation of areas currently occupied by M. tenuis (as in the whole species projection) also is present in this scenario.
The projections for the southern clade (Fig. 2i–l) indicate that, for a clade adapted to the south coast range as a whole, there were frequent periods where the taxon’s range would have been split into an eastern and western range with little to no connectivity between them. This can be seen not only in the MIS19 interglacial (Fig. 2l), but also, surprisingly, in the last glacial maximum (Fig. 2j) when the range expands overall. Even in the present (Fig. 2i), there is an area of reduced habitat suitability in the centre of the range.
The projections for the western subclade (Fig. 2m–p) show a small but very stable range in the southwest corner of South Africa. During the last glacial maximum (Fig. 2n), we see that the range of this clade extends westward along the coast on land exposed by lower sea levels. This could account for the small population of western subclade individuals found within the range of the eastern subclade (Fig. 1e). This population could constitute a relict isolated by changing climate and rising sea levels since the end of the last ice age. Given that most of the Pleistocene has been glaciated with only brief interglacials, the separation of this population from the rest of the western clade may only be occasional and temporary.
The projections for the eastern subclade (Fig. 2q–t) show a reduction in range size during the interglacials, particularly the MIS19 interglacial (Fig. 2t), presumably due to shrinking of the aseasonal rainfall zone. Even during the MIS19 interglacial, however, some suitable habitat remains. This suggests that a subclade adapted to aseasonal rainfall could have persisted in small refugia despite habitat loss during interglacial periods.
The clades and subclades of M. varius are thought to have diverged early on in the Pleistocene: Willows-Munro and Matthee (2011) give a rough estimate of around 2 Ma, with a likely range between 1.75 and 2.7 Ma. As there are no paleoclimatic models available for this time, we used the models available to give us an idea of the extremes in climate experienced during the glacial cycles of the Pleistocene (from about 2.6 Ma). Whilst the last interglacial (130 ka) produced ranges similar to those present today, the MIS19 interglacial (787 ka) resulted in range fragmentation with patterns of vicariance that would potentially result in the current genetic distribution of the species. The MIS19 interglacial itself is too recent to be responsible for the divergence of the (sub)clades; however, the patterns of vicariance produced make interglacials in the early Pleistocene a likely cause of the divergences. The trend seen here is that the more ancient interglacial (MIS19) resulted in greater range contraction and fragmentation than the more recent ones (the last interglacial and the present, which is also an interglacial). However, more data points would be needed to determine whether or not this represents a general trend throughout the Pleistocene.
Based on these data, it seems most likely that the initial population of M. varius (before the divergence of the clades) was similar in its distribution and habitat requirements to the northern clade today. The niche model for the species as a whole was less successful at predicting the current species’ distribution than those for each of the clades, suggesting that the clades are locally adapted. It also is unlikely that a species originating in the north east of South Africa (and ultimately from more tropical populations) would be preadapted for the more Mediterranean climate of the south coast. Furthermore, the model for the northern clade predicts ideal patterns of climate-driven cycles of isolation and connectivity, both from the southern clade and from the range of M. tenuis in the north. This initial M. varius population would have been able to move into the more aseasonal areas along the south coast during glacial periods (Fig. 2f). Such populations then would have become isolated during interglacials (Fig. 2h), promoting the divergence of the clades and the adaptation and spread of the southern clade along the southern coast. This southern clade then would have experienced periodic fragmentation between winter-rainfall areas in the west and aseasonal rainfall areas in the east (Fig. 2i–l), promoting the divergence and local adaptation of the eastern and western subclades. It is worth noting that the clades and subclades of M. varius have experienced little to no genetic mixing since their reciprocal divergence, despite the establishment of secondary contact between them (Willows-Munro and Matthee 2011). Given that the interglacials driving divergence represents only very brief periods during long glacial periods of connectivity, it is likely that this genetic isolation was reinforced by ecologically divergent natural selection. For the Pliocene projections (Fig. 4), the northern clade is used as a proxy for the initial population of M. varius.
Niche model for Myosorex
The niche model for the genus Myosorex performed surprisingly well, considering that it attempted to capture the range of a genus composed of many species that presumably exhibit some variation in environmental tolerances amongst them. The mean AUC value was high and remained well over 0.8 even at the lower range of the standard deviation (0.954 ± 0.061). The range map for the genus (Fig. 3b) fits the location data across most of the range, but loses this correlation somewhat in the north-eastern part of the range. This close fit suggests that the genus inhabits areas with similar environmental conditions across its range, and has been fairly conservative in terms of environmental tolerances and habitat requirements during its radiation. As the location data for Myosorex across Africa are limited, the location data may underrepresent somewhat the range of the genus.
Heat map showing the present suitability of habitat for the genus Myosorex based on the niche modelled by MaxEnt. Hotter colours indicate more suitable habitat. The suitable habitat should match the location data used in model construction (shown as squares). a Map of Africa indicating the geographic location of map b, b Myosorex
The most important variables for the genus as a whole were dry season rainfall (31%), mean annual temperature (28%), mean winter temperature (15%), annual rainfall (8%), mean summer temperature (8%) and winter rainfall (8%). Response curves (Appendix) indicate that the genus is limited to cool, fairly mesic areas with a dry season.
Pliocene projections for Myosorex and the northern clade of M. varius
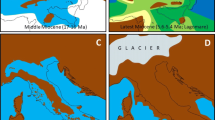
The Pliocene projections for the northern clade of M. varius (Fig. 4a–c) support the idea of divergence from M. tenuis due to climate-induced range fragmentation. During ancient warm periods (represented by the mid-Pliocene warm period), the niche for the northern clade would have retreated south away from the area currently occupied by M. tenuis, whilst during ancient glacial periods (represented by the Pliocene M2 glacial event), the niche would have extended north to connect with it. Myosorex varius and M. tenuis are thought to have diverged around 2.7 Ma, although this divergence could have taken place up to almost a million years earlier or later (Taylor et al. 2013). The ancestral stock of M. varius and M. tenuis diverged from the east coast Myosorex (M. cafer and M. sclateri) around 3.7 Ma (Taylor et al. 2013), and therefore putatively should have been established by the time of the mid-Pliocene warm period, ca. 3.2 Ma.
Heat maps showing the niches of Myosorex varius (northern clade) and Myosorex (entire genus) during climatic extremes of the late Pliocene. Hotter colours indicate more suitable habitat. From left to right: present, mid-Pliocene warm period (3.2 Ma) and the Pliocene M2 glacial event (3.3 Ma). On the northern clade maps (a–c), the current known range of M. tenuis has been circled in red
The Pliocene projections for the northern clade (Fig. 4a–c) also show that, even during the glaciation event, there was a lack of connectivity with quality habitat on the south coast. This conforms to the pattern seen in the Pleistocene projections (Fig. 2) of increasing range reduction and fragmentation in the more distant past. It also leads to the hypothesis that, after the establishment of an initial M. varius population in the area currently occupied by the northern clade, there may have been a delay before the south coast could be colonised. This fits with gap between the estimated divergence times of M. varius and M. tenuis (roughly 2.7 Ma; Taylor et al. 2013) and the northern and southern clades of M. varius (roughly 2 Ma; Willows-Munro and Matthee 2011).
The Pliocene projections for the genus Myosorex (Fig. 4d–f) show that habitats typical of the genus (mesic montane environments in the tropics, spreading into cooler lowland environments in southern Africa) have been strongly affected by climate fluctuations in the past. This suggests that cooler periods provided the opportunity for the genus to ‘island hop’ southward along the string of mountains formed by the Rift Valley from equatorial regions to southern Africa, whilst warmer periods caused species to retreat upslope or further south (in southern Africa), creating highly isolated populations. In the projection for the mid-Pliocene warm period (Fig. 4e), the genus is reduced to a series of small refugia. Interestingly, these refugia closely match the ranges of extant species (Fig. 5).
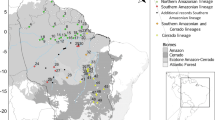
Figure 5 shows the projection for the mid-Pliocene warm period together with the locations of the 19 species of Myosorex based on their respective IUCN red list assessments (Baxter et al. 2020a, b; Cassola 2016; Dando 2021; Engelbrektsson 2016a, b; Engelbrektsson and Kennerley 2020; Gerrie and Kennerley 2019; Kennerley 2016a; b, c, d, e, f, 2019; Kerbis Peterhans and Demos 2021; Plumptre et al. 2019; Taylor and Baxter 2020; Taylor et al. 2021). For M. varius, the locations of the (sub)clades have been included. Myosorex sclateri, M. cafer and M. longicaudatus all occupy small areas of forest and wetland within the range of M. varius. Note that the three species of West Central Africa (M. okuensis, M. rumpii and M. eisentrauti) were not represented in the location data. Because they occur far from the other species and occupy montane areas within the tropical forests of Central Africa, it is likely that they have a somewhat different climatic niche. Nonetheless, M. okuensis is represented by a patch of partly suitable habitat. There are two areas of predicted high suitability that do not correlate with any known living species of Myosorex. One is in the Kenyan highlands (north of M. zinki) and the other is in the Ethiopian highlands (in the far north east of the map). The Kenyan highlands are the home of members of the closely related genus Surdisorex (S. norae and S. polulus) as well as many species of Crocidura (Musila et al. 2019), which may replace Myosorex in this area. Ethiopia is thought to be where the more successful shrew genus Crocidura first appeared (Furió et al. 2007), which could have replaced Myosorex in this area. There are 26 species of Crocidura in Ethiopia, including many forest and montane species (Lavrenchenko et al. 2016). This area also is isolated from Myosorex populations to the south by unsuitable habitat that remained even during the Pliocene glaciation (Fig. 4f). It also is possible that there are undiscovered species of Myosorex in some areas. Although the mid-Pliocene warm period (Fig. 5) represents only a single time period, it demonstrates that the present distribution of the genus can largely be explained by the location of refugia during warm periods.
Discussion
Based on the data presented herein, it appears that the formation of M. varius and its (sub)clades was assisted by climatic fluctuations that caused range expansion during glacial periods and range contraction and fragmentation during warmer interglacials. The most likely evolutionary scenario is that M. varius split off from a common ancestor with M. tenuis towards the end of the Pliocene or around the Pliocene–Pleistocene transition. That initial M. varius population most likely had a distribution similar to that of the northern clade today, and expanded along the south coast to form the current (sub)clades during the early Pleistocene glacial cycles. This scenario also matches up with the estimated divergence times in the literature (Taylor et al. 2013; Willows-Munro and Matthee 2011).
There also is evidence that the (sub)clades of M. varius have undergone local adaptation. First, modelling of (sub)clades separately tended to improve the performance of the models, both in terms of AUC and the ability of projected contemporary niches to match current location data. Second, the (sub)clades occupy distinct climatic niches, based largely on the seasonality of rainfall. The northern clade inhabits mesic, summer-rainfall zones, the western subclade inhabits mesic winter-rainfall zones and the eastern subclade inhabits aseasonal rainfall zones. The eastern subclade in particular has a distribution that so perfectly matches the aseasonal rainfall zone that its niche model can be accurately constructed based on the seasonality of rainfall alone.
Aside from M. varius, other species of Myosorex tend to be lacking in location data, which makes it impossible to construct quality niche models for most of them. The ‘quick and dirty’ method of modelling the genus as a whole, however, yielded surprisingly enlightening results, perhaps due to low levels of ecological diversification within the genus (i.e. most are still limited to cool, mesic montane areas). The general pattern seen in M. varius of range expansion during glacial periods and contraction during warm periods/interglacials also holds true for the genus overall, with cool periods allowing the genus to ‘island hop’ south along the mountains of the East African Rift System towards South Africa. Furthermore, the extant species of the genus are associated with refugia that were predicted to remain suitable even during the mid-Pliocene warm period. A similar pattern of historical persistence in montane refugia has been found for Hylomyscus mice (Rodentia:Muridae:Murinae:Praomyini) and Sylvisorex shrews (Crocidurinae) in the eastern Afromontane regions of the Kenyan Highlands and the Albertine Rift (Demos et al. 2014).
With regard to future climate change, it should be noted that most species within the genus Myosorex already are reduced to ranges within the interglacial montane refugia that previously allowed them to survive the glacial cycles of the past 2.6 million years. These species, therefore, are likely to be highly vulnerable to anthropogenic climate change that takes conditions beyond the range of the glacial cycles they have survived thus far. Of the 19 known species of Myosorex, one (M. eisentrauti) is critically endangered (Kennerley 2016f), seven are endangered (Baxter et al. 2020a; Engelbrektsson 2016b; Kennerley 2016a, d, 2019; Kerbis Peterhans and Demos 2021; Taylor et al. 2021), five are vulnerable (Baxter et al. 2020b; Engelbrektsson 2016a; Engelbrektsson and Kennerley 2020; Kennerley 2016e; Taylor and Baxter 2020), two are data deficient (Kennerley 2016b; Plumptre et al. 2019) and only four are in the least concern category (Cassola 2016; Dando 2021; Gerrie and Kennerley 2019; Kennerley 2016c). It has been estimated that more than half of all data-deficient mammals are threatened with extinction (Borgelt et al. 2022). Given the threat levels of the majority of the species in this genus, it is likely that one or both of the data-deficient Myosorex also are threatened with extinction. Furthermore, according to the same assessments, the critically endangered species, six of the seven endangered species, three of the five vulnerable species, and one of the four least concern species have declining populations. The population trends of the two data-deficient species and one of the least concern species are unknown, whilst the remaining four Myosorex species have stable populations. A recent study assessing the effects of anthropogenic climate change on the five South African Myosorex (Taylor et al. 2017) predicted range reductions for M. varius, M. cafer and M. longicaudatus.
As more location data and more paleoclimatic reconstructions become available, it should become possible to produce quality niche models for more species of Myosorex as well as for time periods more congruent with these species’ estimated times of divergence. Using only the last glacial maximum and last interglacial as an indication of range fluctuations during the glacial cycles of the Pleistocene would have provided little to no evidence of vicariance amongst the clades of M. varius. The more recently produced ‘deep time’ paleoclimates provided evidence of vicariance in more ancient warm periods. Physiological studies comparing the clades of M. varius also would be useful in testing for physiological evidence of local adaptation.
Using palaeoclimate niche modelling, we have shown that Pleistocene glacial cycling can account for the distribution of genetic variation seen in M. varius today. We hypothesise that the current (sub)clades and their distributions are the result of climate-induced vicariance followed by adaptation to local environmental conditions. Palaeoclimatic niche modelling based on earlier climate fluctuations also corroborates the genetically inferred colonisation route of the genus Myosorex along the East African Rift System. The current locations of Myosorex species coincide with climatic refugia that allowed them to survive previous warm periods. As they are already reduced to their ‘warm period refugia’, many of these species are likely to be at serious risk of extinction in the face of further temperature increases due to anthropogenic climate change.
Data availability
All data used in this study are freely available online. The genetic/location data for Myosorex varius are available in the supporting information for Willows-Munro and Matthee (2011). The location data for the rest of Myosorex are available from GBIF.org. The climate data are available from PaleoClim.org.
References
Ashraf U, Peterson AT, Chaudhry MN, Ashraf I, Saqib Z, Rashid Ahmad S, Ali H (2017) Ecological niche model comparison under different climate scenarios: a case study of Olea spp. in Asia. Ecosphere 8:e01825
Baxter R, Willows-Munro S, Taylor P (2020a) Myosorex longicaudatus. The IUCN Red List of Threatened Species 2020. e.T14108A22286725
Baxter R, Taylor PJ, Willows-Munro S (2020b) Myosorex cafer. The IUCN Red List of Threatened Species 2020. e.T110660763A50585251
Bell DM, Schlaepfer DR (2016) On the dangers of model complexity without ecological justification in species distribution modeling. Ecol Model 330:50–59
Borgelt J, Dorber M, Høiberg MA, Verones F (2022) More than half of data deficient species predicted to be threatened by extinction. Commun Biol 5:679
Brown JL, Hill DJ, Dolan AM, Carnaval AC, Haywood AM (2018) PaleoClim, high spatial resolution paleoclimate surfaces for global land areas. Sci Data 5:180254
Cassola F (2016) Myosorex varius. The IUCN Red List of Threatened Species 2016. e.T41382A115519477
Dando T (2021) Myosorex meesteri. The IUCN Red List of Threatened Species 2021. e.T110661822A110662102
Demos TC, Kerbis Peterhans JC, Agwanda B, Hickerson MJ (2014) Uncovering cryptic diversity and refugial persistence among small mammal lineages across the Eastern Afromontane biodiversity hotspot. Mol Phylogenet Evol 71:41–54
Dolan AM, Haywood AM, Hunter SJ, Tindall JC, Dowsett HJ, Hill DJ, Pickering SJ (2015) Modelling the enigmatic late Pliocene glacial event—Marine Isotope Stage M2. Glob Planet Change 128:47–60
Edwards EJ, Osborne CP, Strömberg CAE, Smith SA, C4 Grasses Consortium (2010) The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science 328:587–591
Elith J, Graham CH, Anderson RP, Dudík M, Ferrier S, Guisan A, Hijmans RJ, Huettmann F, Leathwick JR, Lehmann A, Li J, Lohmann LG, Loiselle BA, Manion G, Moritz C, Nakamura M, Nakazawa Y, Overton JMcC, Peterson AT, Phillips SJ, Richardson KS, Scachetti-Pereira R, Schapire RE, Soberón J, Williams S, Wisz MS, Zimmermann NE (2006) Novel methods improve prediction of species’ distributions from occurrence data. Ecography 29:129–151
Engelbrektsson P, Kennerley R (2020) Myosorex jejei (amended version of 2016 assessment). The IUCN Red List of Threatened Species 2020. e.T45954378A166522323
Engelbrektsson P (2016a) Myosorex bururiensis. The IUCN Red List of Threatened Species 2016. e.T45954374A45973041
Engelbrektsson P (2016b) Myosorex gnoskei. The IUCN Red List of Threatened Species 2016. e.T45954382A45973051
ESRI (2017) ArcGIS Release 10.5.1. Redlands, CA
Furió M, Santos-Cubedo A, Minwer-Barakat R, Agustí J (2007) Evolutionary history of the African soricid Myosorex (Insectivore, Mammalia) out of Africa. J Vertebr Paleontol 27:1018–1032
GBIF.org (2021) Global biodiversity information facility. https://www.gbif.org
Gerrie R, Kennerley R (2019) Myosorex babaulti. The IUCN Red List of Threatened Species 2019. e.T41380A22287111
Goulden EA, Meester J (1978) Notes on the behaviour of Crocidura and Myosorex (Mammalia: Soricidae) in captivity. Mammalia 42:197–208
Happold M, Happold DCD (eds) (2013) Mammals of Africa, vol IV. Hedgehogs, shrews and bats. Bloomsbury Publishing, London
Heikkinen RK, Marmion M, Luoto M (2012) Does the interpolation accuracy of species distribution models come at the expense of transferability? Ecography 35:276–288
Hill DJ (2015) The non-analogue nature of Pliocene temperature gradients. Earth Planet Sci Lett 425:232–241
IUCN (2022) The IUCN Red List of Threatened Species, Version 2022-1 [WWW Document]. https://www.iucnredlist.org. Accessed 15 Oct 2022
Karger DN, Conrad O, Böhner J, Kawohl T, Kreft H, Soria-Auza RW, Zimmermann NE, Linder P, Kessler M (2017) Climatologies at high resolution for the Earth land surface areas. Sci Data 4:170122. https://doi.org/10.1038/sdata.2017.122. Repository: Dryad Digital Repository https://doi.org/10.5061/dryad.kd1d4
Kennerley R (2016a) Myosorex rumpii. The IUCN Red List of Threatened Species 2016. e.T14113A115120529
Kennerley R (2016b) Myosorex schalleri. The IUCN Red List of Threatened Species 2016. e.T14110A115120282
Kennerley R (2016c) Myosorex zinki. The IUCN Red List of Threatened Species 2016. e.T45048A115201876
Kennerley R (2016d) Myosorex geata. The IUCN Red List of Threatened Species 2016. e.T14107A115120122
Kennerley R (2016e) Myosorex okuensis. The IUCN Red List of Threatened Species 2016. e.T14112A115120383
Kennerley R (2016f) Myosorex eisentrauti. The IUCN Red List of Threatened Species 2016. e.T14106A22286815
Kennerley R (2019) Myosorex kihaulei. The IUCN Red List of Threatened Species 2019. e.T45047A22287518
Kerbis Peterhans JC, Huhndorf MH, Plumptre AJ, Hutterer R, Kaleme P, Ndara B (2008) Mammals, other than bats, from the Misotshi-Kabogo highlands (eastern Democratic Republic of Congo) with the description of two new species (Mammalia: Soricidae). Bonn Zool Bull 62:203–219
Kerbis Peterhans JC, Hutterer R, Mwanga J, Ndara B, Davenport L, Karhagomba IB, Udelhoven J (2010) African shrews endemic to the Albertine Rift: two new species of Myosorex (Mammalia: Soricidae) from Burundi and the Democratic Republic of Congo. J East Afr Nat Hist 99:103–128
Kerbis Peterhans J, Demos T (2021) Myosorex blarina. The IUCN Red List of Threatened Species 2021. e.T14111A22286334
Lavrenchenko LA, Voyta L, Hutterer R (2016) Diversity of shrews in Ethiopia, with the description of two new species of Crocidura (Mammalia: Lipotyphla: Soricidae). Zootaxa 4196:038–060
Levinsky I, Araújo MB, Nogués-Bravo D, Haywood AM, Valdes PJ, Rahbek C (2013) Climate envelope models suggest spatio-temporal co-occurrence of refugia of African birds and mammals. Glob Ecol Biogeog 22:351–363
Li X, Wang Y (2013) Applying various algorithms for species distribution modelling. Integr Zool 8:124–135
Meynard CN, Gay P-E, Lecoq M, Foucart A, Piou C, Chapuis M-P (2017) Climate-driven geographic distribution of the desert locust during recession periods: subspecies’ niche differentiation and relative risks under scenarios of climate change. Glob Change Biol 23:4739–4749
Musila S, Monadjem A, Webala PW, Patterson BD, Hutterer R, De Jong YA, Butynski TM, Mwangi G, Chen Z-Z, Jiang X-L (2019) An annotated checklist of mammals of Kenya. Zool Res 40:3–52
Neumann FH, Bamford MK (2015) Shaping of modern southern African biomes: Neogene vegetation and climate changes. Trans R Soc S Afr 70:195–212
Otto-Bliesner BL, Marshall SJ, Overpeck JT, Miller GH, Hu A, CAPE Last Interglacial Project Members (2006) Simulating Arctic climate warmth and icefield retreat in the last interglaciation. Science 311:1751–1753
Pahad G, Montgelard C, Jansen van Vuuren B (2020) Phylogeography and niche modelling: reciprocal enlightenment. Mammalia 84:10–25
Partridge TC, Scott L, Hamilton JE (1999) Synthetic reconstructions of southern African environments during the Last Glacial Maximum (21–18 kyr) and the Holocene Altithermal (8–6 kyr). Quat Int 57(58):207–214
Peterson AT (2001) Predicting species’ geographic distributions based on ecological niche modelling. Condor 103:599–605
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modelling of species geographic distributions. Ecol Model 190:231–259
Phillips SJ, Dudík M, Schapire RE (2004) A maximum entropy approach to species distribution modelling. In: Proceedings of twenty-first Int Conf Mach Learn, pp 655–662
Plumptre A, Dando T, Kennerley R (2019) Myosorex kabogoensis. The IUCN Red List of Threatened Species 2019. e.T112042073A112042093
Quérouil S, Hutterer R, Barrière P, Colyn M, Kerbis Peterhans JC, Verheyen E (2001) Phylogeny and evolution of African shrews (Mammalia: Soricidae) inferred from 16s rRNA sequences. Mol Phylogenet Evol 20:185–195
Ramírez-Preciado RP, Gasca-Pineda J, Arteaga MC (2019) Effects of global warming on the potential distribution ranges of six Quercus species (Fagaceae). Flora 251:32–38
Skinner JD, Chimimba CT (2005) The mammals of the southern African sub-region. Cambridge University Press, Cambridge
Sosdian S, Rosenthal Y (2009) Deep-sea temperature and ice volume changes across the Pliocene–Pleistocene climate transitions. Science 325:306–310
Taylor P, Baxter R (2020) Myosorex sclateri. The IUCN Red List of Threatened Species 2020. e.T14114A22287210
Taylor PJ, Kearney TC, Peterhans JCK, Baxter RM, Willows-Munro S (2013) Cryptic diversity in forest shrews of the genus Myosorex from southern Africa, with the description of a new species and comments on Myosorex tenuis. Zool J Linn Soc 169:881–902
Taylor PJ, Willows-Munro S, Baxter R, Monadjem A, Child MF (2016) A conservation assessment of Myosorex varius. In: Child MF, Roxburgh L, Do Linh San E, Raimondo D, Davies-Mostert HT (eds) The red list of mammals of South Africa, Swaziland and Lesotho. South African National Biodiversity Institute and Endangered Wildlife Trust, Pretoria
Taylor PJ, Ogony L, Ogola J, Baxter R (2017) South African mouse shrews (Myosorex) feel the heat: using species distribution models (SDMs) and IUCN Red List criteria to flag extinction risks due to climate change. Mammal Res 62:149–162
Taylor P, Willows-Munro S, Baxter R, Monadjem A, Child MF (2021) Myosorex tenuis. The IUCN Red List of Threatened Species 2021. e.T110662121A22287436
Tognelli MF, Roig-Juñent SA, Marvaldi AE, Flores GE, Lobo JM (2009) An evaluation of methods for modelling distribution of Patagonian insects. Rev Chil de Hist Nat 82:347–360
Verboom GA, Archibald JK, Bakker FT, Bellstedt DU, Conrad F, Dreyer LL, Forest F, Galley C, Goldblatt P, Henning JF, Mummenhoff K, Linder HP, Muasya AM, Oberlander KC, Savolainen V, Snijman DA, van der Niet T, Nowell TL (2009) Origin and diversification of the Greater Cape flora: ancient species repository, hot-bed of recent radiation, or both? Mol Phylogenet Evol 51:44–53
Waltari E, Guralnick RP (2009) Ecological niche modelling of montane mammals in the Great Basin, North America: examining past and present connectivity of species across basins and ranges. J Biogeogr 36:148–161
Waltari E, Hijmans RJ, Peterson AT, Nyári ÁS, Perkins SL, Guralnick RP (2007) Locating Pleistocene refugia: comparing phylogeographic and ecological niche model predictions. PLoS One 2:e563
Willows-Munro S, Matthee CA (2009) The evolution of the southern African members of the shrew genus Myosorex: understanding the origin and diversification of a morphologically cryptic group. Mol Phylogenet Evol 51:394–398
Willows-Munro S, Matthee CA (2011) Linking lineage diversification to climate and habitat heterogeneity: phylogeography of the southern African shrew Myosorex varius. J Biogeogr 38:1976–1991
Acknowledgements
The authors acknowledge S. Willows-Munro and C. A. Matthee for their work on the phylogeography of Myosorex varius (Willows-Munro and Matthee 2011) as well as the Global Biodiversity Information Facility (GBIF.org) and the PaleoClim online database (PaleoClim.org).
Funding
Open access funding provided by University of Johannesburg.
Author information
Authors and Affiliations
Contributions
GP: niche modelling, first draft of manuscript and editing. BJvV: supervision. CM: review and editing.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling editor: Luis A. Ruedas.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pahad, G., Jansen van Vuuren, B. & Montgelard, C. Palaeoclimatic niche modelling reveals the phylogeographic history of the South African shrew Myosorex varius and the colonisation route of the genus Myosorex (Mammalia, Soricidae) from East Africa. Mamm Biol 103, 579–590 (2023). https://doi.org/10.1007/s42991-023-00377-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42991-023-00377-0