Abstract
Assessing the spatiotemporal behaviour of alien species is pivotal to designing effective management plans. Interspecific niche partitioning among ungulates is reported as a strategy to avoid direct interactions. The Mediterranean mouflon and wild boar are two ungulates introduced to Elba island for hunting and aesthetic purposes. We used intensive camera trapping to test whether species occupancy and temporal activity rhythms would vary in response to the presence or absence of the co-occurring species through multi-species occupancy modelling. Our findings report a lack of spatial and temporal segregation between the two species for the late spring–summer and late summer–autumn seasons. In contrast, results for the winter–early spring period suggest that spatial partitioning between wild boar and mouflon is present in areas with high artificial cover (e.g., paved roads). Animals may indeed exploit roads to move more rapidly in search of food; however, their occurrence in these areas seems to be influenced by the presence of the other species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interspecific interactions, such as competition and ecological niche partitioning, play a key role in shaping ecological communities and species spatiotemporal distribution. When two or more species interact, one can directly or indirectly affect the other (Connell 1983), both at the individual and population levels, by altering the ecological processes that determine competitor species fitness—including habitat use, activity rhythm, and feeding behaviour (MacArthur 1972; Ferretti and Mori 2020). For this reason, understanding the nature and the extent of these interactions is essential in implementing effective wildlife management and conservation practices (Connell 1983; Miller et al. 2017).
Competition arises from the combinations of the three following conditions: (1) the presence of different species sharing the same resources, (2) limited resources, and (3) the emergence of negative effects following the joint exploitation of resources (Minle 1961; Prins 2000; Pascual-Rico et al. 2020).
Co-existence among co-evolved competing species is made possible by their ability to fill different ecological niches; this process, known as niche partitioning, is the result of the exploitation of resources and conditions that are shaped by the interactions between species over time (Wright 2002; Grassel et al. 2015; Ballejo et al. 2018; Pascual-Rico et al. 2020). In addition, Hutchinson (1957) argues that “niche partitioning” can occur on different dimensions, including dietary requirements, habitat use and temporal distribution (Botella 2020).
The introduction of non-native species beyond their natural range can trigger novel intraguild competition that, in turn, can have far-reaching consequences for the complex web of relations that determine the natural functioning of ecosystems (Weller et al. 2011; Donihue et al. 2020). Despite the considerable progress in the study of biological invasions, much remains to be understood on the dynamics and effects of co-occurring non-native species (Grosholz et al. 2000; Crooks 2002; Johnson et al. 2009).
Interactions among non-native species can generate three possible outcomes (Rauschert and Shea 2017): positive (i.e., invasional meltdown—a species invasion is facilitated and/or its impacts are made worse by the presence of another non-native species, see Simberloff and Von Holle 1999), neutral (i.e., species invasions may not impact or be independent of each other: Jackson 2015), or negative (i.e., invasional interference—the invasion of one species may be impeded or obstacle by the presence of another non-native species: Yang et al. 2011; Rauschert and Shea 2012, 2017). As the number of non-native species increases, it becomes ever more imperative to assess the ecological consequences of biological interactions for the integrity of natural communities (Vitousek et al. 1997; Kuebbing et al. 2013).
The Mediterranean mouflon Ovis aries musimon (hereafter, mouflon) is a feral subspecies of domestic sheep that originated in the three Mediterranean islands of Sardinia and Corsica (Hermans 1996), which is currently under intensive conservation management to reduce the risk of localised extinctions (c.f. Hadjisterkotis 2001). However, it was introduced into other regions of Europe for recreational and ornamental purposes in the twentieth century (Gippoliti et al. 2006). Following the establishment of viable populations, increasing grazing pressure has caused considerable damage to both agriculture and natural systems (Guidi et al. 2009). It is now the object of population control programmes (Chiatante et al. 2013).
On the contrary, the wild boar Sus scrofa is a widespread species native to much of Eurasia and North Africa and shows a strong ability to adapt to different environmental conditions (Lowe et al., 2000; Barrios-García and Ballari 2012). The species is now considered a major conservation concern in numerous natural systems in Europe and farther afield (Lowe 2004; Gürtler et al. 2017). The main drivers for the rapid spread of wild boar in recent decades are represented by the extirpation of natural predators, habitat modification, and the progressive abandonment of the countryside (Gamelon et al. 2012; Massei et al. 2015; Vetter et al. 2015; Gürtler et al. 2017).
The introduction of the species to new areas in response to sport hunting and further recreation interests has raised concerns among ecologists and conservationists for the detrimental repercussions for the local biodiversity (Lowe et al., 2000; Barrios-García and Ballari 2012; Greco et al. 2020).
Both wild boar and mouflon were introduced into the offshore island of Elba, part of the Tuscan Archipelago National Park in central Italy (hereafter, TANP), during the second-half of the twentieth century (De Marinis et al. 1996; Boitani et al. 2003; Angelici et al. 2009; Meriggi et al. 2015; Greco et al. 2020), where, under favourable habitat conditions and in the absence of predators, both have established self-sustaining populations. Furthermore, the combination of rooting, grazing, browsing and treading by these large, introduced animals represents, amongst others, a primary threat to the local vegetation, which in turn is known to be an essential resource for endemic animal species (see Cini et al. 2021). To the best of our knowledge, however, there has been no study on the ecological implications of the co-occurrence of wild boar and mouflon on the protected ecosystem of Elba.
While direct interspecific competition in the form of resource exclusion remains to be assessed and is beyond the scope of this research, previous studies have reportedly confirmed negative interactions between wild boar and mouflon. In this regard, calves and lamb predation by wild boar has been observed in the wild (Bratton 1974; Plant 1997). Further negative interactions may stem from the tendency of wild boar to soil waterholes that mouflon uses for drinking, with the additional possibility of disease transmission that can further hinder the coexistence of the two species (Hadjisterkotis 2001). Finally, potential competition over food arises when and where resources are limited (Hadjisterkotis 2001). Understanding the nature of the interactions is critical for implementing effective non-native species management programmes (c.f. Kuebbing and Nuñez 2015; Jackson 2015). In particular, the asynchronous removal of species may either favour or oppose the expansion of the other with yet unknown repercussions for the island ecosystem.
In addition, reported damages to the island's agriculture and natural system have prompted the park’s authorities to promote the eradication of the two non-native species (TANP 2010). In this regard, by focusing on the study of niche partition along two main dimensions—namely, spatial and temporal segregation—the current research aims to investigate the extent and nature of the interactions between wild boar and mouflon in Elba. The aim is to form a baseline to inform TANP authorities on the likely effects of population control management practices on the occurrence of the two species and, therefore, the repercussions for the ecosystem.
Using 1 year of camera-trap data (i.e., April 2018–2019) collected over three consecutive sampling seasons in TANP, we investigated whether and to what extent wild boar and mouflon interactions influence their occurrence at the spatial and temporal dimensions.
To this aim, we first applied the multi-species occupancy modelling approach developed by Rota et al. (2016) to assess whether spatial segregation exists between the two species. We drew from the conclusions of Greco et al. (2020) on wild boar occurrence on Elba, and Ferretti and Mori (2020) on ungulate species interactions, to derive three broad categories of environmental variables to be used in our modelling:
-
1.
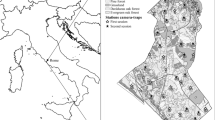
Habitat: Greco et al. (2020) showed that a key limiting factor for the occurrence of wild boar in the study area is food availability; building from this finding, we expected that, if segregation exists, the two species will tend to occupy habitats characterised by different vegetation structures (e.g., different resources). In addition, as wild boar has been recorded to select areas with rich deciduous forests (Melis et al. 2006; Meriggi et al. 2015; Greco et al. 2020) and ecotones (Fattorini and Ferretti 2020; Ferretti et al. 2021), we anticipated that, in case of segregation, mouflon occupancy would be the lowest in these habitats, which are predominantly found along the northern slope aspect of mount Capanne (Fig. 1), i.e., in mostly unsuitable areas for the wild boar (cf. Ferretti et al. 2021).
-
2.
Human: we predicted that mouflons would occupy areas with high proportions of artificial cover to avoid encounters with the wild boar, especially in spring, as documented in other ungulate species (e.g., Ferretti et al. 2011). These could include human settlements, inland industrial sites, or infrastructures, where they would experience greater human disturbance but lower encounters with the other species (Chirichella et al. 2013; Ohashi et al. 2013; Palupe et al. 2016; Blehyl et al. 2019). We also decided to include distance to human settlements to test whether proximity to urban landscapes can influence species occurrence.
-
3.
Elevation: as wild boar is expected to occupy areas at lower altitudes, where water and vegetation cover are more abundant, we expected mouflon to occupy regions at higher altitudes of mount Capanne, which occupies the westernmost sector of the island (Foggi et al. 2006; Meriggi et al. 2015; Greco et al. 2020).
Second, we assessed whether temporal segregation occurs between wild boar and mouflon, especially during the breeding season, to limit the potential risk of negative interspecific interactions. Temporal partitioning has been observed in other taxa as a common coping strategy adopted by co-occurring species (Hayward and Slotow 2009). According to Greco et al. (2020), the wild boar population on Elba is predominantly nocturnal; we, therefore, hypothesized that mouflons would display a diurnal activity pattern, possibly to reduce encounters with wild boar. In addition, as for other primarily diurnal species (Carnevali et al. 2016; Grignolio et al. 2018), the nocturnal activity of wild boar mainly occurs on bright moonlight nights to better detect natural predators (Brivio et al. 2017; Gaudiano et al. 2022, but see Gordigiani et al. 2022). With this in mind, we also sought to assess whether patterns of activity rhythms between wild boar and mouflon would vary in response to moon phases.
Materials and methods
Study area
The research was conducted in the Mount Capanne (1019 m a.s.l.) area on the western sector of Elba Island (42° 46′20.4''N, 10° 10′14.4''E), part of the TANP in central Italy (see Greco et al. 2020; Mori et al. 2021, for the location of the study site and camera trap stations). Deciduous woodlands are restricted to the northern slope, whereas Mediterranean maquis and garrigue (Quercus ilex L., Pistacia lentiscus L., Salvia rosmarinus L., Lavandula stoechas L., Erica arborea L., Arbutus unedo L. and Cistus spp.) occupy the southern slopes. As a result of reforestation policies after the Second World War, patches of pine plantations (mostly Pinus pinea L., Pinus pinaster Aiton and Pseudotsuga menziesii (Mirb.) Franco) are also present along the mountain slopes (Foggi et al. 2006). Villages and cultivations (including orchards and vineyards) occur outside the borders of the TANP, while a major paved road connects villages around the park borders (Greco et al. 2020). The climate is predominantly Mediterranean (annual average T, 17 °C; mean annual rainfall, 95 mm), with dry summers and mild winters and sporadic snowfall events restricted to the summit of Mount Capanne. Terrestrial mammal species in the study area include several small rodents and shrew species, wild boar Sus scrofa, European mouflon Ovis aries, Italian hare Lepus corsicanus, European brown hare Lepus europaeus and pine marten Martes martes (Greco et al. 2020; Mori et al. 2021).
Sampling design
Fieldwork was conducted over three seasons to cover an entire solar year (cf. Greco et al. 2020; Mori et al. 2021): 7th April–15th July 2018 (spring–summer); 1st September–18th November 2018 (late summer–autumn), and 18th January–8th April 2019 (winter–early spring), season duration varied slightly according to field team availability. During each season, 80 stations covering an area of about 2878 ha were sampled using 20 unbaited, motion-triggered camera traps deployed in four consecutive arrays for an average of 18 days each (Mori et al. 2021). Three different brands of camera trap (hereafter CT), similar in technical characteristics (e.g., IR flash and 0.8–1 s trigger speed), were used, including Ltl Acorn–Shenzhen, Guangdong, China; Spromise–Shenzhen, Guangdong, China; and U-way—Atlanta, Georgia, USA. To provide relatively unbiased measures of species distribution across environmental gradients (i.e., elevation and vegetation composition), CTs were run in a systematic design, from the low elevation points to the peak along with both sectors of mount Capanne (Fig. 1). In other words, the first location was selected randomly, and the other were placed at fixed distances (Mori et al. 2021). Due to the terrain's harshness and the dense vegetation characterising the study area, CTs were placed along trekking trails, about 20 m off-trails, following the altitudinal gradient, at a set distance of at least 500 m from each other (Fig. 1). CTs were secured to tree trunks or rocks at about 50 cm from the ground level, near signs of wildlife such as scats and tracks (see Greco et al. 2020). During each visit, we collected landscape data to estimate the probability of occupancy and species detection. These included percentages of tree cover (plants taller than 5 m), shrub cover (plants less than 5 m in height), herbaceous cover (herbaceous plants) recorded within 10 m radius from the CT station, elevation (recorded using Garmin GPS Etrex 32X), and mountain aspect (i.e., north–south, see Mori et al. 2021). Eight CT stations were moved within similar habitat conditions after the first season due to the inaccessibility of the original locations' terrain, which had limited on-site operations (Fig. 1). Across seasons, CTs were placed within a buffer area of 20 m2 off the selected point to minimize errors in the sampling design.
Detection and occupancy covariates
To explore if and how wild boar and mouflon spatially segregate, we used three categories of environmental and anthropogenic factors (Table 1) to fit multi-species occupancy models implemented in “unmarked” (Fiske and Chandler 2011):
-
1)
Habitat: we used the Corine Land Cover Classification System (hereafter C.L.C.; European Environment Agency, 2018) (20 m resolution) to derive eight non-mutually exclusive percentage classes of habitat types within 100 m in from the CT (Table 1). These included “artificial surfaces” (C.L.C. 1—level 1, including urban, industrial, construction and commercial sites, mines, and non-agricultural vegetated areas), “agricultural areas” (C.L.C. 2—level 1, permanent crops and arable lad), “broad-leaved forests” (C.L.C. 311—level 3), “coniferous forests” (C.L.C. 312—level 3), “mixed forests” (C.L.C. 313—level 3), “sclerophyllous vegetation” (C.L.C. 323—level 3), “transitional woodland-shrub” (C.L.C. 324—level 3), and “bare rocks” (C.L.C. 332—level 3). Based on the ecological requirements of wild boar (see Greco et al. 2020), we considered “natural” areas (i.e., C.L.C. 311–324) to represent suitable habitats; therefore, segregation would restrict mouflon occurrence to other habitats in the study area. We also included the slope aspect of mount Capanne (north–south), as varying solar radiation intensity can shape optimal microhabitat characteristics for which competition may arise between the two species (Greco et al. 2020; Måren et al. 2015).
-
2)
Anthropogenic disturbance: we used metric distance to the closest human settlement (QGis Development Team 2019, 1:10,000 scale map) as a proxy for human pressure (Table 1). While most urban centres are located along the coast, human density fluctuates noticeably throughout the year, reaching its peak in summer when the island becomes a popular destination among tourists. Because of this, we expected human disturbance to have a stronger influence on species occurrence during the first sampling season (i.e., late spring–summer).
-
3)
Elevation, which was measured in m a.s.l., using Garmin GPS Etrex 32X, at each camera trap station during ground-truthing of the study area (Table 1).
In addition, we studied the effects of camera-trap brand and vegetation cover in the 10 m surrounding the CTs (see Sampling design session) on the detectability of the species (Table 1). CT brands may differ slightly in reaction times and affect the probability of recording target species (Greco et al. 2020; Rovero et al. 2013), whereas thicker vegetation may conceal animals (Gu and Swihart 2002; Apps and McNutt 2018; Greco et al. 2020).
Data analysis
Camera-trap records were annotated and stored in Wild.ID (Fegraus and MacCarthy 2016), which allows for species identification using the IUCN nomenclature (Greco et al. 2020). From the resultant file, we extracted the number of independent events (set at 30 min intervals) and estimated raw descriptors of species capture, including relative abundance index (as the ratio of events to the sampling effort) and naïve occupancy (proportion of sites occupied by the target species on sites sampled). Each season was normalised to have a duration of a maximum of 18 days per CT array: photos taken after the end of the 18th day of operation were not considered for the analysis.
We used the multi-species occupancy modelling approach described by Rota et al. (2016), a generalization of MacKenzie et al. (2002), to research the co-occurrence effects of wild boar and mouflons. To do this, we exploited the function occuMulti implemented in “unmarked” developed by Fiske and Chandler (2011), using R (version 3.6.3., R Foundation for Statistical Computing, Vienna, Austria: cran.r-project.org). Each season was independently analysed. The advantage of following this approach is twofold: first, it enabled us to cope with the effects of the decision to move eight CTs after the first season, and second, it allowed us to evaluate the effects of seasonal changes in environmental and anthropogenic pressure variables on species detectability and occupancy measures. As per Greco et al. (2020) and Mori et al. (2021), detection/non-detection data were arranged in \(i\times j\) matrices of sites by surveys (i.e., sampling occasions), with each entry indicating the presence (1) or absence (0) of species in site i on survey j. These matrices were then used as input for the downstream analysis of detection and occupancy. Continuous covariates were standardised to have a mean of 0 and SD equals 1 (Rovero and Zimmermann 2016). Covariates that exhibited a Pearson’s r greater than 0.6 were considered collinear and were not included in the same models (Table 2).
In addition to a null model (i.e., assuming constant p and \(\Psi\)), we first built models for the detection (p) while holding occupancy probability constant (\(\psi =1\); see MacKenzie et al. 2002, 2006; Johnson et al. 2020). Constant occupancy models’ fitness was assessed by ranking the candidate models according to the Akaike Information Criterion (AIC). Conditionally on the best detection structure (sensu Mori et al. 2021), we assessed environmental variables’ effects on species occupancy (Greco et al. 2020; Mori et al. 2021). We analyzed two sets of occupancy models (Burnham and Anderson 2002), which were later integrated into the same analytic framework for cross-model comparison (Miller et al. 2018). The first entailed marginal occupancy models, where mouflons and wild boar were treated as two independently occurring species. The second comprised conditional occupancy models that assumed variability in wild boar and mouflons’ occupancy probability in response to the presence or absence (Rota et al. 2016; Miller et al. 2018). This way, we could assess the extent of interspecific interactions between wild boar and mouflons. To build marginal and conditional occupancy models for wild boar and mouflon, we used the same covariates for the two species and all possible combinations, except for collinear variables not included within the same model. Using a cutoff value for \(\Delta AIC\) of 2, models with \(\Delta AIC\le 2\) were considered statistically supported and were used to derive a prediction for \(\Psi\) and p. Variables for which credible intervals (CI) did not intersect 0 were considered good environmental predictors and were plotted in R using ggplot2 (Wickham 2016).
Patterns of activity rhythms
We reported the date and the solar hour of capture directly shown on each photo on a Microsoft Excel data set, including all records. The use of the solar hour allows a better evaluation of activity patterns as, differently from the “legal hour”, it is defined by the sun's position in the sky regardless of local time, which varies across seasons. We defined activity as the cumulative period animals spend while non-resting, regardless of their behaviour (Mori et al. 2020a, b). We limited the pseudoreplication bias by removing records of the same species occurring in the same camera trap site within < 30 min (Mori et al. 2020a, b). The only wild boar activity patterns have previously been analyzed for the same sampling periods and in the same study site (Greco et al. 2020). Instead, patterns of seasonal activity rhythms of the mouflon were analyzed through the R package “overlap” (Meredith and Ridout 2014). The overlap coefficient of temporal activity patterns (Δ) between the mouflon and the wild boar was computed during annual and seasonal periods. Particularly, we used the Δ1 estimator when records for at least one species in each pairwise comparison of our analyses were less than 75, Δ4 when records of both species were more than 75, in line with previous literature and as suggested by the R package instructions (Meredith and Ridout 2014; Monterroso et al. 2014). We considered as “moderate” a Δ value included between 0.50 and 0.75, “high” when it was > 0.75 and “very high” when delta > 0.90 (Mazza et al. 2019). We then estimated the 95% confidence intervals (hereafter, CI) for Δ, as percentile intervals from 999 bootstrap samples (Monterroso et al. 2014). Bootstrap tests were used to obtain a probability test that two sets of circular observations belonged to the same distribution with the function compareCkern() of the R-package activity (Havmøller et al. 2020). The Mardia–Watson–Wheeler test (MWW) was calculated with the R package circular to compare overlapping circadian distribution of mouflons and wild boar among different seasons (Lund et al. 2017). The Hermans–Rasson test (r test) was used to estimate whether the mouflon exhibited a random activity pattern and the wild boar round-the-clock (Landler et al. 2019). A chi-squared test was used to estimate whether records of each ungulate species were uniformly distributed throughout all the four moon phases, classified as follows: phase 1: from new moon to ¼; phase 2: from ¼ to ½; phase 3: from ½ to ¾; phase 4: over ¾.
Results
A total of 9 CTs were either stolen or damaged over the three different periods and, therefore, generated no data, while others switched off before the 18th day; this leads to a cumulative sampling effort of 4,137 days, yielding a total of 943 independent events for the mouflon (spring–summer, N = 231; late summer–autumn, N = 456; winter–early spring, N = 256) and 587 for the wild boar (spring–summer, N = 88; late summer–autumn, N = 355; winter–early spring, N = 144) (Table 3). Other species detected during the study period included pine martens, European brown hares, domestic cats, domestic dogs and humans.
Occupancy analysis
Our analysis of the detection of the two species over the three sampling seasons showed little variation throughout the year. Different vegetation cover types consistently improved model fit in the three seasons of samplings (Table 4). In particular, we confirmed that increasing tree cover had a positive effect on wild boar detection during season 1 (\({p}_{wb}\pm SE= 0.47 \pm 0.18 , P<0.05\); with 95% CI between 0.17 and 0.77) (Fig. 2a), whereas shrub cover percentage correlated negatively with mouflon detectability (\({p}_{m}\pm SE= - 0.349 \pm\) 0.10, \(P<0.05\); with 95% CI between − 0.51 and − 0.18) (Fig. 2b). Herbaceous cover negatively affected the probabilities of detection for both species in the second season (\({p}_{wb}\pm SE= - 0.52 \pm 0.13, P<0.05\); with 95% CI between − 0.73 and − 0.31 and\({p}_{m}\pm SE= - 0.29 \pm 0.09, P<0.05\); with 95% CI between − 0.45 and − 0.13) (Fig. 3a, b). Finally, we recorded a negative impact of herbaceous cover on wild boar detection in the third season as well (\({p}_{wb}\pm SE= - 0.48 \pm 0.16\), \(P<0.05\); with 95% CI between − 0.75 and − 0.22) (Fig. 4a).
a Predicted wild boar occupancy in response to bare rocks cover (C.L.C. 332), b predicted mouflon occupancy in response to sclerophyllous vegetation cover (C.L.C. 323), c predicted mouflon occupancy in response to distance to settlements, d predicted conditional occupancy of mouflon in relation to artificial surfaces and in response to wild boar presence and absence during sampling season 3
By feeding this information into the analysis of marginal and conditional occupancy of wild boar and mouflon for the three seasons (Table 4), we found that:
in season 1, no covariate was found to be a strong predictor of marginal and conditional occupancy of wild boar and mouflon during the first sampling season, CIs intersect zero.
In season 2, similar to season 1, marginal and conditional occupancy of the two species appeared to have no significant relation with the variables analyzed in this study (Table 4).
In season 3, top-ranked models (i.e., with \(\Delta AIC<2\)) for marginal occupancy showed variation with three covariates (Fig. 4). The wild boar was less likely to occupy areas covered in bare rocks (C.L.C. 332; \({\Psi }_{wb} \pm SE\)= − 2.22 \(\pm\) 1.007\(, P<0.05\); with 95% CI between − 3.87 and − 0.56) (Fig. 4a), whereas mouflon occupancy decreased, where sclerophyllous vegetation was more abundant (C.L.C. 323; \({\Psi }_{m} \pm SE\)= − 0.69 \(\pm\) 0.344, \(P<0.05\); with 95% CI between − 0.95 and − 0.02) (Fig. 4c) but increased with proximity to human settlements (\({\Psi }_{m} \pm SE\)= -0.87 \(\pm\) 0.416, \(P<0.05\); with 95% CI between − 1.08 and − 0.072) (Fig. 4c). However, results from model-cross comparisons between marginal and conditional occupancy showed that the top-ranking model, with the lowest \(\Delta\)AIC, was the conditional model \(f{m}_{63}\) (Table 4 and Fig. 4d). This provided strong evidence of occurrence dependence between the two species during the final season of sampling when we estimated lower mouflon occupancy in areas with a high proportion of artificial cover given the presence of wild boar (\({\Psi }_{m | wb }\pm SE = - 2.01\pm 0.84, P<0.05\); with 95% CI between − 3.39 and − 0.64). Top ranking model predictions of marginal and conditional occupancy of wild boar and mouflon relative to the three sampling seasons are reported in Table S1.
Patterns of activity rhythms of the mouflon and overlap with the wild boar
Pairwise inter-seasonal overlaps of the mouflon were all moderate (Fig. 5; Table 5). Pairwise inter-seasonal differences were not significant, i.e., both analysed sets of circular observations come from the same distribution (all P > 0.05) and all analysed overlaps were similar one-another (MWW test: 0.002 < W < 0.173; P > 0.10).
Pairwise overlaps between mouflon and wild boar were moderate or high in all seasons (Fig. 6). Both analysed sets of circular observations come from the same distribution (all P > 0.05) and all analysed overlaps were similar one-another (MWW test: 0.002 < W < 0.173; P > 0.10). A non-random activity pattern round-the-clock was exhibited throughout the year by the wild boar and the mouflon, with main peaks at dawn and dusk (Hermans–Rasson tests: R = 198.4–203.1; P < 0.001).
Intra-seasonal activity overlap of the mouflon and the wild boar in Elba Island was assessed through kernel density estimates throughout our sampling periods. Coefficients of temporal overlap and 95% CIs of mouflon and wild boar seasonal activity patterns in Elba island are shown. Black rectangles indicate dark hours
We detected no effect of moon phases on the activity of both mouflons
(χ2 = 3.14–5.08, df = 3, P > 0.05) and wild boar (χ2 = 1.16–2.87, df = 3, P > 0.05) in any season.
Discussion
This work represents the first study on the co-occurrence of two alien ungulates on Elba island. The ecology of wild boar and Mediterranean mouflon has long been a focal point of scientific research, with particular attention to the repercussions for ecological communities as they alter ecosystem processes and functions (Barrios-García and Ballari 2012; Celesti-Grapow et al. 2017). Studies have also highlighted significant socio-economic issues related to the rate at which these species can colonise new environments (Volery et al. 2020).
Spatial predictions for wild boar and mouflon occurrence on Elba showed no evidence of segregation amongst species. Ranking of the multi-species occupancy models consistently revealed that neither marginal nor the conditional occupancy were affected by any of the analysed variables. However, the apparent spatial segregation documented during the winter suggests that wild boar and mouflon tend to avoid each other during the colder months in areas with relatively high artificial surface cover. This category (C.L.C. Artificial surfaces—Level I) includes paved roads, which are commonly used by animals to move more efficiently in search for food as confirmed by similar studies (Portanier et al. 2018).
In addition, for the winter period, our results showed that wild boar tends to avoid areas with comparatively high bare rock cover during winter. This corroborates our expectations and previous findings (Greco et al. 2020) that the species distribution is limited by the availability of resources and lower resource-poor habitats, such as areas with large expanses of bare rocks. On the other hand, mouflons were found to avoid areas with a high abundance of sclerophyllous vegetation while showing a preference for sites close to human settlements. While this behaviour requires additional investigations, our suggestion is that animals may be allured by salt licks made available by residents during the colder months (residents, personal communication, August 2019). This supports earlier results showing an increasing tendency among wild mammals to use human villages as shelters during demanding environmental conditions (Santini et al. 2019). However, the relatively high abundance of human settlements along the coast, and hence at lower altitudes (as shown by the strong correlation between the two variables; Table 2), represents a confounding factor. Therefore, we may suggest that mouflons occurred at lower altitudes during the colder months to avoid harsh weather conditions and benefit from a more abundant resource availability.
As to activity rhythms, the mouflon confirmed a marked bimodal activity pattern, with activity peaks occurring at dawn and dusk, in line with previous literature on both radio-tagged (Pipia et al. 2008; Bourgoin et al. 2011) and camera-trapped mouflons (Centore et al. 2018). Despite a high interseasonal overlap of activity rhythms of this species, the dawn activity peak occurred earlier in the warmest month of the year. In contrast, the dusk activity peak was delayed, in line with the photoperiod (Bourgoin et al. 2011). A decrease in diurnal activity in warm months represents an adaptation to reduce thermoregulation costs (Bourgoin et al. 2008). However, our data have not recorded this activity reduction and may only represent an adaptation in mountain ecosystems (Bourgoin et al. 2008). Accordingly, in Mediterranean areas, mouflons (particularly lactating females) may show the greatest activity levels in summer to fulfil their need to meet greater energetic demands, e.g., for lactation (Ciuti et al. 2009).
Similarly, in natural and undisturbed conditions, the wild boar is active both during the day and the night, with alternating periods of activity and rest, with activity peaks at dawn and dusk (Podgórski et al. 2013; Brivio et al. 2017; Mori et al. 2020a, b). In human-dominated landscapes, including also rural and suburban areas, the wild boar is primarily nocturnal (Brivio et al. 2017; Rossa et al. 2021; Gordigiani et al. 2022), independently of the seasonal changes in day length to reduce interference with humans (Keuling et al. 2013). Seasonal variation in the activity patterns is low (Keuling et al. 2013; Mori et al. 2020a, b). In our data set, both ungulate species showed significant activity peaks at dawn and dusk throughout all seasons, showing no temporal niche partitioning. Furthermore, we observed no effect of the moon phase on the nocturnal activity of both species. Nocturnal activity of herbivore species is reported as a compensatory opportunity for energy intake when activity in daylight is too dangerous because of humans or predators (Visscher et al. 2017; Grignolio et al. 2018).
In many cases, prey species tend to avoid bright moonlight, although several ungulate species increase their activity on brightest nights when their ability to detect predators is the highest (Medici 2010; Brown et al. 2011). Similarly, when natural predators are present, the nocturnal activity of the wild boar mainly occurs on bright moonlight nights, when environmental lighting should be the highest, mainly where natural predators occur (Brivio et al. 2017). Conversely, our results fit with Johann et al. (2020), who detected no effect of the moon phase on activity patterns in rural areas, where natural predators are absent. As to the mouflon, no data are available on the effect of the moon phase on activity in the presence of predators. Further research is needed to assess whether this species also adapts to the moon phase.
While coherent conclusions are hindered by the limited amount of data at our disposal (1 year only), we urge the relevant authority to consider the possible implications of species population control practices. Accordingly, the reported absence of niche partitioning, as indicated by the multi-species modelling ranking results for the first two sampling periods, suggests that there might be a neutral interaction between wild boar and mouflon. Although a neutral interaction is likely to be due either to different feeding requirements (and hence different ecological niches) or to the availability of resources on the island, additional studies are needed to define better the diet composition and resource requirements for the two non-native species. This might help shed light on the interactions between wild boar and mouflon on the island of Elba and, therefore, better understand how population control plans can affect their occurrence and ecological impacts on the island.
Data availability
Data used in this study are available on reasonable request via the corresponding author.
References
Angelici FM, Laurenti A, Nappi A (2009) A checklist of the mammals of small Italian islands. Hystrix 20:3–27
Apps P, McNutt JW (2018) Are camera traps fit for purpose? A rigorous, reproducible and realistic test of camera trap performance. Afr J Ecol 56:710–720
Ballejo F, Lambertucci SA, Trejo A, De Santis LJ (2018) Trophic niche overlap among scavengers in Patagonia supports the condor-vulture competition hypothesis. Bird Conserv Intern 28(3):390–402
Barrios-García MN, Ballari SA (2012) Impact of wild boar (Sus scrofa) in its introduced and native range: a review. Biol Invasions 14:2283–2300
Bleyhl B, Arakelyan M, Askerov E, Bluhm H, Gavashelishvili A, Ghasabian M, Ghoddousi A, Heidelberg A, Khorozyan I, Malkhasyan A, Manvelyan K, Masoud M, Moqnaki EM, Radeloff VC, Soofi M, Weinberg P, Zazanashvili N, Kuemmerle T (2019) Assessing niche overlap between domestic and threatened wild sheep to identify conservation priority areas. Divers Distrib 25:129–141
Boitani L, Lovari S, Vigna-Taglianti A. (2003) Fauna d’Italia. Mammalia III—Carnivora, Artiodactyla. Edizioni Calderini, Bologna, Italy.
Bourgoin G, Garel M, Van Moorter B, Dubray D, Maillard D, Marty E, Gaillard JM (2008) Determinants of seasonal variation in activity patterns of mouflon. Can J Zool 86:1410–1418
Bourgoin G, Garel M, Blanchard P, Dubray D, Maillard D, Gaillard JM (2011) Daily responses of mouflon (Ovis gmelini musimon × Ovis sp.) activity to summer climatic conditions. Can J Zool 89:765–773
Bratton SP (1974) The effect of the European wild boar (Sus scrofa) on the high-elevation vernal flora in Great Smoky Mountains National Park. Bull Torr Botanic Club. 101(4):198–206
Brivio F, Grignolio S, Brogi R, Benazzi M, Bertolucci C, Apollonio M (2017) An analysis of intrinsic and extrinsic factors affecting the activity of a nocturnal species: the wild boar. Mammal Biol 84:73–81
Brown B, Bryntesson F, Cooper S, Nyholm B, Robertson D, Bedford A, Hendricks D, Klippenstein L, Potapov E (2011) Moonlight and suburban white-tailed deer movements. Bull New Jersey Ac Sci 56:1–4
Burnham KP, Anderson DR (2002) Model selection and inference—a practical information-theoretic approach. Springer Editions, New York, USA
Carnevali L, Lovari S, Monaco A, Mori E (2016) Nocturnal activity of a “diurnal” species, the northern chamois, in a predator-free Alpine area. Behav Processes 126:101–107
Celeste-Grapow L, Abbate G, Baccetti N, Capizzi D, Carli E, Copiz R. (2017) Control of invasive species for the conservation of biodiversity in Mediterranean islands. The LIFE PonDerat project in the Pontine Archipelago, Italy. Plant Biosystems—An international Journal Dealing with all Aspects of Plant Biology. Springer, New York, USA.
Centore L, Ugarković D, Scaravelli D, Safner T, Pandurić K, Šprem N (2018) Locomotor activity pattern of two recently introduced non-native ungulate species in a Mediterranean habitat. Folia Zool 67:17–24
Chiatante G, Meriggi A, Giustini D, Baldaccini NE (2013) Density and habitat requirements of red-legged partridge on Elba Island (Tuscan archipelago, Italy). Ital J Zoology 80(3):402–411
Chirichella R, Ciuti S, Apollonio M (2013) Effects of livestock and non-native mouflon on use of high-elevation pastures by Alpine chamois. Mamm Biol 78:344–435
Cini A, Benetello F, Platania L, Bordoni A, Boschi S, Franci E, Ghisolfi G, Pasquali L, Negroni R, Dapporto L (2021) A sunny spot: habitat management through vegetation cuts increases oviposition in abandoned fields in an endemic Mediterranean butterfly. Insect Conserv Div 14(5):582–596
Ciuti S, Pipia A, Grignolio S, Ghiandai F, Apollonio M (2009) Space use, habitat selection and activity patterns of female Sardinian mouflon (Ovis orientalis musimon) during the lambing season. Eur J Wildl Res 55:589–595
Connell J (1983) On the prevalence and relative importance of interspecific competition—Evidence from field experiments. Am Nat 122:661–696
Crooks JA (2002) Characterizing ecosystem-level consequences of biological invasions: the role of ecosystem engineers. Oikos 97:153–166
De Marinis AM, Masseti M, Sforzi A (1996) Note on the non-flying terrestrial mammals of the Tuscan Archipelago, Northern Tyrrhenian Sea (Italy). Boll Mus Reg Sci Nat Torino 14:275–281
Donihue CM, Daltry JC, Challenger S, Herrel A (2020) Population increase and changes in behavior and morphology in the Critically Endangered Redonda ground lizard (Pholidoscelis atratus) following the successful removal of alien rats and goats. Integr Zool 16:379–389
Fattorini N, Ferretti F (2020) Estimating wild boar density and rooting activity in a Mediterranean protected area. Mamm Biol 100:241–251
Fegraus E, MacCharty J (2016) Camera trap data management and interoperability. In: Rovero F, Zimmermann F (eds) Camera trapping for wildlife research. Pelagic Publisher, Exeter, UK
Ferretti F, Sforzi A, Lovari S (2011) Behavioural interference between ungulate species: roe are not on velvet with fallow deer. Behav Ecol Sociobiol 65:875–887
Ferretti F, Lovari S, Lucherini M, Hayward M, Stephens PA (2020) Only the largest terrestrial carnivores increase their dietary breadth with increasing prey richness. Mammal Rev 50:291–303
Ferretti F, Mori E (2020) Displacement interference between wild ungulate species: does it occur? Ethol Ecol Evol 32:2–15
Ferretti F, Lazzeri L, Mori E, Cesaretti G, Calosi M, Burrini L, Fattorini N (2021) Habitat correlates of wild boar density and rooting along an environmental gradient. J Mammal 102:1536–1547
Fiske I, Chandler R (2011) Unmarked: an R package for fitting hierarchical models of wildlife occurrence and abundance. J Stat Softw 43:1–23
Foggi B, Cartei L, Pignotti L, Signorini MA, Viciani D (2006) Il paesaggio vegetale dell’Isola d’Elba (Arcipelago Toscano): studio fitosociologico e cartografico. Fitosociol 43:3–94
Gamelon M, Gaillard JM, Servanty S, Gimenez O, Toïgo C, Baubet E, Klein F, Lebreton JD (2012) Making use of harvest information to examine alternative management scenarios: a body weight-structured model for wild boar. J Appl Ecol 49(4):833–841
Gaudiano L, Pucciarelli L, Frassanito AG, Mori E, Morimando F, Silvestri FM, Sorino R, Viviano A, Corriero G (2022) Spatio-temporal behaviour of female wild boar in an agro-forestry-pastoral landscape of Southern Italy. Mammal Res 67:163–172
Gippoliti S, Amori G (2006) Ancient introductions of mammals in the Mediterranean Basin and their implications for conservation. Mammal Rev 36(1):37–48
Gordigiani L, Viviano A, Brivio F, Grignolio S, Lazzeri L, Marcon A, Mori E (2022) Carried away by a moonlight shadow: activity of wild boar in relation to nocturnal light intensity. Mammal Res 67:39–49
Grassel SM, Rachlow JL, Williams CJ (2015) Spatial interactions between sympatric carnivores: asymmetric avoidance of an intraguild predator. Ecol Evol 5(14):2762–2773
Greco I, Fedele E, Salvatori M, Giampaoli Rustichelli M, Mercuri F, Santini G, Rovero F, Lazzaro L, Foggi B, Massolo A, De Pietro F, Zaccaroni M (2020) Guest or pest? Spatio-temporal occurrence and efforts on soil and vegetation of the wild boar on the Elba Island. Mammal Biol 101:193–206
Grignolio S, Brivio F, Apollonio M, Frigato E, Tettamanti F, Filli F, Bertolucci C (2018) Is nocturnal activity compensatory in chamois? A study of activity in a cathemeral ungulate. Mamm Biol 93:173–181
Grosholz ED, Ruiz GM, Dean CA, Shirley KA, Maron JL, Connors PG (2000) The impacts of a nonindigenous marine predator in a California bay. Ecol 81:1206–1224
Gu W, Swihart RK (2002) Absent or undetected? Effects of non-detection of species occurrence on wildlife-habitat model. Biol Conserv 116:195–203
Guidi T, Foggi B, Arru S, Lazzaro L, Giannini F (2009) Effects of grazer populations on the woody vegetation of the islands of Capraia and Elba (Tuscan Archipelago-Livorno). Georgofili. 6:97–119
Gürtler RE, Martín Izquierdo V, Gil G, Cavicchia M, Maranta A (2017) Coping with wild boar in a conservation area: impacts of a 10-year management control program in north-eastern Argentina. Biol Invasions 19(1):11–24
Hadjisterkotis E, Nahlik A, Uloth W. 2001 The Cyprus mouflon, a threatened species in a biodiversity “hotspot” area. In Proceedings of the Third International Symposium on Mouflon. Sopron, Hungary: Institute of Wildlife Management. 71–81
Havmøller RW, Jacobsen NS, Scharff N, Rovero F, Zimmermann F (2020) Assessing the activity pattern overlap among leopards (Panthera pardus), potential prey and competitors in a complex landscape in Tanzania. J Zool. https://doi.org/10.1111/jzo.12774
Hayward MW, Slotow R (2009) Temporal partitioning of activity in large African carnivores: tests of multiple hypotheses. S Afr J Wildl Res 39:109–125
Hermans WA (1996) De Europese moeflon, Ovis musimon [The European mouflon, Ovis musimon]. Tijdschr Diergeneeskd 121(18):515–517 (Dutch. PMID: 8928183)
Hutchinson GE (1957) January. Population studies-animal ecology and demography-concluding remarks. Cold Spring Harbor Symposia Quantitative Biology. 22:415–427
Jackson MC (2015) Interactions among multiple invasive animals. Ecology 96:2035–2041
Johann F, Handschuh M, Linderoth P, Heurich M, Dormann CF, Arnold J (2020) Variability of daily space use in wild boar Sus scrofa. Wildl Biol. https://doi.org/10.2981/wlb.00609
Johnson PTJ, Olden JD, Solomon CT, Vander Zanden MJ (2009) Interactions among invaders: community and ecosystem effects of multiple invasive species in an experimental aquatic system. Oecologia 159:161–170
Johnson CL, Hilser H, Linkie M, Rahasia R, Rovero F, Pusparini W, Hunowu I, Patandung A, Andayani N, Tasirin J, Nistyantara LA, Bowkett AE (2020) Using occupancy-based camera-trap surveys to assess the critically endangered primate Macaca nigra across its range in North Sulawesi, Indonesia. Oryx 54:784–793
Kery M, Royle JA. (2020) Applied Hierarchical Modeling in Ecology: Analysis of Distribution, Abundance and Species Richness in R and BUGS: Volume 2: Dynamic and Advanced Models. Academic Press, London, UK.
Keuling O, Baubet E, Duscher A, Ebert C, Fischer C, Monaco A, Podgorski T, Prevot C, Ronnenberg K, Sodeikat G, Stier N, Thurfjell H (2013) Mortality rates of wild boar Sus scrofa L. in central Europe. Eur J Wildl Res 59:805–814
Kuebbing SE, Nuñez MA, Simberloff D (2013) Current mismatch between research and conservation efforts: the need to study co-occurring invasive plant species. Biol Cons 160:121–129
Kuebbing SE, Nuñez MA (2015) Negative, neutral, and positive interactions among nonnative plants: patterns, processes, and management implications. Glob Change Biol 21(2):926–934
Landler L, Ruxton GD, Malkemper EP (2019) The Hermans-Rasson test as a powerful alternative to the Rayleigh test for circular statistics in biology. BMC Ecol 19:1–8
Lowe S, Browne M, Boudjelas S, De Poorter M (2000) 100 of the world’s worst invasive alien species: a selection from the global invasive species database, vol 12. Invasive Species Specialist Group, Auckland
Lund U, Agostinelli C, Arai H, Gagliardi A, Portugues EG, Giunchi D, Irisson JO, Pocernich M. Rotolo F (2017) Package circular. http://mirrors.ucr.ac.cr/CRAN/ web/packages/circular/circular.pdf. Accessed on 03.12.2020.
Macarthur R (1972) Geographical ecology: patterns in the distribution of species. Princeton University Press, Princeton, NJ
Mackenzie DI, Nichols JD, Lachman GB, Droege S, Royle JA, Langtimm CA (2002) Estimating site occupancy rates when detection probabilities are less than one. Ecology 83:2248–2255
MacKenzie DI, Nichols JD, Royle JA, Pollock KH, Bailey LL, Hines JE (2006) Occupancy estimation and modeling. Elsevier Academic Press Editions, Burlington, Massachusetts, USA, Inferring patterns and dynamics of species occurrence
Måren IE, Karki S, Prajapati C, Yadav RK, Shrestha BB (2015) Facing north or south: does slope aspect impact forest stand characteristics and soil properties in a semiarid trans-Himalayan valley? J Arid Environ 121:112–123
Massei G, Genov PV, Staines BW (1996) Diet, food availability and reproduction of wild boar in a Mediterranean coastal area. Acta Theriol 41:307–320
Massei G, Kindberg J, Licoppe A, Gačić D, Šprem N, Kamler J, Baubet E, Hohmann U, Monaco A, Ozoliņš J, Cellina S (2015) Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest Manag Sci 71(4):492–500
Mazerolle MJ, Mazerolle MMJ (2019) Package AICcmodavg. R package version 2.2–2. https://cran.uib.no/web/packages/AICcmodavg/AICcmodavg.pdf. Accessed on 06.12.2020.
Mazza G, Marraccini D, Mori E, Priori S, Marianelli L, Roversi PF, Gargani E (2020) Assessment of color response and activity rhythms of the invasive black planthopper Ricania speculum (Walker, 1851) using sticky traps. Bull Entomol Res 110:480–486
Medici EP (2010) Assessing the viability of lowland tapir populations in a fragmented landscape. Dissertation, University of Kent Canterbury, UK.
Melis C, Szafrańska PA, Jędrzejewska B, Bartoń K (2006) Biogeographical variation in the population density of wild boar (Sus scrofa) in western Eurasia. J Biogeogr 33:803–811
Meredith M, Ridout M (2014) Overview of the Overlap Package. Available from: http://cran.cs.wwu.edu/web/packages/overlap/vignettes/overlap.pdf. Accessed on 02.12.2020.
Meriggi A, Lombardini M, Milanesi P, Brangi A, Lamberti P, Giannini F (2015) Management of wild boar in protected areas: the case of Elba Island. In: Angelici FM (ed) Problematic Wildlife. A cross-disciplinary approach, Springer, New York, pp 229–251
Miller JRB, Pitman RT, Mann GKH, Fuller AK, Balme GA (2018) Lions and leopards coexist without spatial, temporal or demographic effects of interspecific competition. J Anim Ecol 87:1709–1726
Milne A (1961) Definition of competition among animals. Cambridge Univ. Press, Cambridge
Monterroso P, Alves PC, Ferreras P (2014) Plasticity in circadian activity patterns of mesocarnivores in Southwestern Europe: implications for species coexistence. Behav Ecol Sociobiol 68:1403–1417
Mori E, Andreoni A, Cecere F, Magi M, Lazzeri L (2020a) Patterns of activity rhythms of invasive coypus Myocastor coypus inferred through camera-trapping. Mammal Biol 100:591–599
Mori E, Bagnato S, Serroni P, Sangiuliano A, Rotondaro F, Marchianò V, Cascini V, Poerio L, Ferretti F (2020b) Spatiotemporal mechanisms of coexistence in an European mammal community in a protected area of southern Italy. J Zool 310:232–245
Mori E, Fedele E, Greco I, Giampaoli Rustichelli M, Massolo A, Miniati S, Puppo F, Santini G, Zaccaroni M (2021) Spatiotemporal activity of the pine marten Martes martes: insights from an island population. Ecol Res. https://doi.org/10.1111/1440-1703.12269
Ohashi H, Saito M, Hori R, Tsunoda H, Noba H, Ishi H, Kuwabara T, Hiroshige Y, Koike S, Hoshino T, Toda Y, Kaji K (2013) Differences in the activity pattern of the wild boar Sus scrofa related to human disturbance. Eur J Wildl Res 59:167–177
Palomares F, Caro TM (1999) Interspecific killing among mammalian carnivores. Am Nat 153:492–508
Palupe B, Leopold CR, Hess SC, Faford JK, Pacheco D, Judge SW (2016) Changes in habitat use and distribution of mouflon in the Kahuku Unit of Hawai ‘i Volcanoes National Park. Pacific Conserv Biol 22:308–311
Pascual-Rico R, Sánchez-Zapata JA, Navarro J, Eguía S, Anadón JD, Botella F (2020) Ecological niche overlap between co-occurring native and exotic ungulates: insights for a conservation conflict. Biol Invasions 22:2497–2508
Pipia A, Ciuti S, Grignolio S, Luchetti S, Madau R, Apollonio M (2008) Influence of sex, season, temperature and reproductive status on daily activity patterns in Sardinian mouflon (Ovis orientalis musimon). Behaviour 145:1723–1745
Plant JW, Rees D, Marchant RS, Mitchell TD, 1977. Feral pigs, predators of lambs [New South Wales, sheep]. Agricultural Gazette of New South Wales.
Podgórski T, Baś G, Jędrzejewska B, Sönnichsen L, Śnieżko S, Jędrzejewski W, Okarma H (2013) Spatiotemporal behavioral plasticity of wild boar (Sus scrofa) under contrasting conditions of human pressure: primeval forest and metropolitan area. J Mammal 94:109–119
Portanier E, Larroque J, Garel M, Marchand P, Maillard D, Bourgoin G, Devillard S (2018) Landscape genetics matches with behavioral ecology and brings new insight on the functional connectivity in Mediterranean mouflon. Landsc Ecol 33:1069–1085
Prins HH (2000) Competition between wildlife and livestock in Africa. Wildlife conservation by sustainable use. Springer, Netherlands, Dordrecht, pp 51–80
Rauschert ESJ, Shea K (2012) Invasional interference due to similar inter- and intraspecific competition between invaders may affect management. Ecol Appl 22:1413–1420
Rauschert ESJ, Shea, (2017) Competition between two similar invasive species: modeling invasional interference across a landscape. Popul Ecol 59:79–88
Rossa M, Lovari S, Ferretti F (2021) Spatiotemporal patterns of wolf, mesocarnivores and prey in a Mediterranean area. Behav Ecol Sociobiol 75:1–13
Rota CT, Ferreira MAR, Kays R, Forrester TD, Kalies EL, McShea WJ, Parsons AW, Millspaugh JJ (2016) A multispecies occupancy model for two or more interacting species. Meth Ecol Evol 7:1164–1173
Rovero F, Zimmermann F, Berzi D, Meek P (2013) “Which camera trap type and how many I need?” A review of camera features and study design for a range of wildlife research applications. Hystrix 24:148–156
Rovero F, Zimmermann F (2016) Camera trapping for wildlife research. Pelagic Publishing Ltd, Exeter, UK
Santini L, González-Suárez M, Russo D, Gonzalez-Voyer A, von Hardenberg A, Ancillotto L (2019) One strategy does not fit all: determinants of urban adaptation in mammals. Ecol Lett 22:365–376
Simberloff V, Von Holle B (1999) Positive interactions of nonindigenous species: invasional meltdown? Biol Invasions 1:21–32
Tuscan Archipelago National Park (TANP) (2010) Capitolato tecnico per il servizio di prelievo ungulati nel Parco Nazionale Arcipelago Toscano periodo 2018–2020. CIG 746127788E. Technical Report. Available at: https://www.islepark.it/attachments/article/1351/Capitolato%20tecnico.pdf (In Italian)
Vetter SG, Ruf T, Bieber C, Arnold W (2015) What is a mild winter? Regional differences in within-species responses to climate change. PLoS ONE 10(7):e0132178
Visscher DR, Macleod I, Vujnovic K, Vujnovic D, Dewitt PD (2017) Human risk induced behavioral shifts in refuge use by elk in an agricultural matrix. Wildl Soc Bull 41:162–169
Vitousek PM, Mooney HA, Lubchenco J, Melillo JM (1997) Human domination of Earth’s ecosystems. Science 277:494–499
Volery L, Jatavallabhula D, Scillitani L, Bertolino S, Bacher S (2020) Ranking alien species based on their risks of causing environmental impacts: a global assessment of alien ungulates. Global Change Biol. https://doi.org/10.1111/gcb.15467
Weller SG, Cabin RJ, Lorence DH, Perlman S, Wood K, Flynn T, Sakai AK (2011) Alien plant invasions, introduced ungulates, and alternative states in a mesic forest in Hawaii. Restor Ecol 19:671–680
Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis. Springer, New York
Wright JS (2002) Plant diversity in tropical forests: a review of mechanisms of species coexistence. Oecologia 130(1):1–14
Yang SA, Ferrari MJ, Shea K (2011) Pollinator behavior mediates negative interactions between two congeneric invasive plant species. Am Nat 177:110–118
Acknowledgements
The authors would like to thank I. Greco, who helped in fieldwork, A. Massolo, who kindly provided camera traps, and the Tuscan Archipelago National Park personnel. We would like to thank the Associate Editor Francesco Ferretti and two anonymous reviewers, who kindly took the time to improve our manuscript with their comments.
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
MZ, GS and FG: conceived and designed the experiments. EF, MGR, FDS and MM: performed the experiments. EM and EF: analyzed the data. EM, EF and MZ: wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling editor: Francesco Ferretti.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fedele, E., Mori, E., Giampaoli Rustichelli, M. et al. Alien versus alien: spatiotemporal overlaps among introduced ungulates in a Mediterranean island ecosystem. Mamm Biol 102, 1981–1995 (2022). https://doi.org/10.1007/s42991-022-00313-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42991-022-00313-8