Abstract
The technology for the activation of irrigation brackish water was successful at improving the soil environment and crop yields. However, very few studies have examined how activated brackish water irrigation affects the rhizosphere bacterial communities and network patterns. By combining 16S rRNA high-throughput sequencing, we evaluated the diversity and composition of the rhizosphere bacterial community after being subjected to different types of irrigation with activated brackish water. We also analyzed the correlation and co-occurrence networks among the bacterial diversity, composition, and rhizosphere soil properties. The results showed that compared with brackish water irrigation, the salt content of activated irrigation water significantly decreased by 9.35%, 9.83%, and 12.95%, respectively. Irrigation with different types of activated brackish water had no significant (p > 0.05) effect on the diversity of the rhizosphere bacterial community, but it significantly changed its community composition, which was primarily dominated by soil nutrient indicators. The soil total nitrogen (TN) showed a significant (p < 0.01) negative correlation with the Chao1 index. Additionally, the changes in bacterial communities under different types of activated brackish irrigation water mainly occurred at the genus level. We showed that the rhizosphere soil that had been irrigated with oxygenated brackish water and magnetized brackish water better supported the reproduction of some soil-borne pathogens. Magnetization-oxygenation coupling treatment could significantly reduce the colonization of soil-borne pathogens of the rhizosphere soil, while also favoring the function of functional bacteria involved in soil nutrient transformation. This study highlights the main factors affecting the rhizosphere soil bacterial community structure by activated brackish water irrigation, while also providing new technical support for brackish water irrigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The imbalance between the supply and demand of freshwater resources is not only an important bottleneck for agricultural development (Aivazidou and Tsolakis 2021) but also an important factor that affects the stability of the agricultural ecosystems (Li et al. 2018a, b). Under conditions of limited water resources, providing more efficient agricultural irrigation and improving the water use efficiency are important issues that require urgent action. To solve this imbalance, countries worldwide have considered developing and utilizing inferior water as an alternative to overcome the shortage of freshwater resources. Despite brackish water resources in China being widely distributed and largely available, they are still underutilized. The development of irrigation using brackish water has significant potential. Although irrigation with brackish water meets the water requirements of crops during different periods (Cheng et al. 2021), their long-term use causes secondary salinization of the soil, deteriorates the soil’s ecological environment, and reduces the yield and quality of crops (Liu et al. 2019a, b; Wei et al. 2021). Therefore, combining the rational and efficient development of inferior water resources with the prevention and control of soil salinization along with a reduction in the salinity-induced stress on crop growth is vital for developing water-efficient irrigation and realizing green and sustainable agricultural development.
Activated water technology involves physically treating irrigation water to significantly change its physical and chemical properties, so as to improve the activity of irrigation water (Wang et al. 2019). Recently, irrigation water activation technology, like magnetization and oxygenation, are simple techniques that have low-energy consumption and low input costs and are pollution-free and efficient for treating water, thus making them a hot research topic (Zhang et al. 2022; Zhu et al. 2021). Improving the physical and chemical characteristics of irrigation water boosts its physiological efficacy, which further enhances the efficient transmission capacity of irrigation water from the soil to crops (Wei et al. 2022). The physiological production potential of irrigation water in agricultural production is exploited to improve the comprehensive efficacy of irrigation water in the agricultural ecosystem to render the activated water technology an important approach in agricultural water-saving and efficiency-increasing measures.
Previous studies have shown that both oxygenated and magnetized water irrigation technology can effectively improve the efficiency of producing irrigation water to promote crop production (Abd Elhady et al. 2021; Hozayn et al. 2016; Zhao et al. 2018a, b). Oxygenated irrigation technology can increase the soil oxygen content by either increasing the dissolved oxygen content of the irrigation water or directly delivering oxygen to the crop roots (Hou et al. 2021). It can promote the growth and development of the crop roots and improve their root activity and absorption capacity (Bhattarai et al. 2008; Yafuso and Fisher 2018). Moreover, it can affect the activities and community structure of soil microorganisms (Cui et al. 2020; Zhao et al. 2018a, b), thereby enhancing the absorption and utilization of water and nutrients by the crop roots and improving their water use efficiency. Additionally, the development of magnetized water treatment technology also made the brackish water application feasible in agricultural irrigation. The magnetic field can change the infrared and ultraviolet absorption intensity of irrigation water (Pang and Deng 2008), which subsequently affects the macroscopic characteristics of water, like surface tension, pH, viscosity, electrical conductivity, oxygen content, and salt solubility (Moosavi and Gholizadeh 2014). Studies have shown that as compared with ordinary fresh water, magnetized freshwater irrigation significantly increased the seed germination rate, promoted seedling growth (Morejón et al. 2007; Zhang et al. 2022), and improved the nutrient absorption and utilization of crops (Radhakrishnan and Ranjitha 2012). Furthermore, it can enhance photosynthesis by increasing the leaf photosynthetic pigments to significantly improve the crop yield and quality (Hozayn et al. 2016, 2019). In terms of crop water use efficiency, magnetized water can change the water biofilm permeability and affect the water absorption of crops, thereby improving their utilization efficiency of irrigation water (Liu et al. 2019a, b). Moreover, the magnetized water irrigation-induced changes in the soil’s physical and chemical properties primarily manifest as a reduction in the soil salt content, an increase in the soil water content, an increased organic matter decomposition, and an increase in the soil nutrient content (Al-Ogaidi et al. 2017; Cui et al. 2020; Zhou et al. 2021a, b).
Irrigation with oxygenated and magnetized water could affect the soil’s bacterial activities (Lu 2018; Zhao et al. 2018a, b), thereby enhancing the absorption and utilization of water and nutrients by the crop roots and also improving the crop’s water and fertilizer utilization efficiency. Although the oxygen availability could affect the activity of anammox bacteria, the anaerobic ammonium oxidation process is considered to be an important aspect of nitrogen (N) loss, which is limited by the activity of anammox bacteria and the oxygen concentration. Currently, most of the discovered anammox bacteria belong to the Planctomyces genus (Dedysh et al. 2020). Studies have shown that appropriate soil dissolved oxygen concentration favors the propagation of anammox bacteria (Planctomyces), but a too high concentration inhibits their growth (Fu et al. 2020). Furthermore, maintaining the appropriate soil dissolved oxygen concentration could also increase the activity of nitrifying bacteria, reduce the activity of anaerobic microorganisms like denitrifying bacteria, and subsequently improve the available N content of rhizosphere soil (Fan et al. 2013; Pan et al. 2015). Additionally, the N fixation process is crucial for maintaining the biological productivity of ecosystems, because it can compensate for the bioavailable N loss caused by anammox and denitrification (Capone and Knapp 2007). The appropriate soil dissolved oxygen concentration in the soil is crucial for the N fixation process of rhizobia (Jiang et al. 2021). For example, rhizobia need sufficient energy to meet the energy required for respiration to complete the N fixation process. However, nitrogenase, essential for rhizobial N fixation, could only function under low-oxygen conditions. Magnetization or oxygenation treatment could increase the dissolved oxygen concentration in the irrigation water (Zhang et al. 2022; Zhu et al. 2021), which might affect the soil bacterial activity and consequently the soil N transformation process. For example, magnetized irrigation water could change the soil bacterial community structure, especially increase the relative abundance of Proteobacteria, Firmicutes, and Bacteroidetes, and was closely related to the contents of soil ammonium N, nitrate N, and organic matter (Cui et al. 2020). However, the enrichment of soil (rhizosphere) N could also affect the activity of anammox bacteria. Irrigation using magnetized or oxygenated water could improve the rhizospheric soil N nutrition status (rhizosphere) (Ke et al. 2015; Mghaiouini et al. 2021), which indirectly promotes the soil bacterial growth by stimulating plant productivity (Wardle et al. 2004). In general, the soil bacteria have a high N demand but they also exhibit stoichiometric steady-state, thereby indicating that N fixation is dependent on bacterial growth (Hicks et al. 2020). Moreover, water could activate the activity of anammox bacteria in the arid soil, which would significantly increase with the increase of soil nitrate concentration (Wang et al. 2020). Therefore, magnetized water or oxygenated irrigation water could affect the soil bacterial community structure and diversity by changing the soil dissolved oxygen concentration or its nutrient status.
However, most studies to date have focused separately on either oxygenation or magnetized freshwater irrigation, with only a few reports on the impact of the coupling effect of oxygenation and magnetization on the soil bacterial community structure, particularly when combined with brackish water irrigation. Therefore, we aim to evaluate the effects of irrigation with magnetized-oxygenated brackish water on the soil’s bacterial diversity and community structure. We have proposed the following hypotheses: (1) There are significant differences in the soil bacterial community structure following different irrigation treatments with activated brackish water, and (2) the magnetization-oxygenation coupling treatment could reduce the pathogenic bacterial colonization of rhizosphere soil while maintaining the lower soil salinity and better soil moisture conditions.
2 Materials and Methods
2.1 Test Soil
The test soil sample was saline-alkaline soil (0–20 cm) in the surface layer of the experimental field of the test station of Xinjiang Bazhou Water Conservancy Administration (CITY, China). Its initial soil salt content was 4.79 g kg−1, with the pH being 8.07. The organic matter content, TN, nitrate N, available phosphorus (P), and available potassium (K) were 7.22 g kg−1, 0.51 mg kg−1, 4.50 mg kg−1, 20.60 mg kg−1, and 139.50 mg kg−1, respectively. According to Kaczynski’s soil particle classification standard and the use of a laser particle size analyzer (Mastersizer 2000, Malvern Instruments Co., Ltd., Malvern, UK), the contents of physical clay, powder, and sand in the soil sample were 2.94%, 32.54%, and 64.50%, respectively. It was medium loam soil.
2.2 Experimental Method and Design
The test brackish water was prepared using NaCl (content ≥ 99.5%, analytical pure AR) and freshwater (dissolved oxygen concentration of 8–10 mg L−1). The experiment was established with four treatments: brackish water (CK), magnetized brackish water (M), oxygenated brackish water (O), and magnetized-oxygenated brackish water (MO). The mean values of the pH, salinity, dissolved oxygen concentration, and the surface tension of different activated brackish water types are presented in Table 1.
A micro-nanobubble rapid generator (TL-HP20-A + ; China) was used to oxygenate the brackish water. During the oxygenation process, an HQ40 portable dissolved oxygen meter (Seven2Go™; Mettler Toledo, Shanghai, China) was used to monitor the dissolved oxygen concentration of the brackish water. An external 3000 GS permanent magnet (CHQ; Baotou Xinda Magnetic Material Factory, Baotou, China) was used for the magnetization treatment. The magnetization-oxygenation coupling treatment involved magnetizing and subsequent oxygenation using the abovementioned instruments.
The pot used for soil culture had a diameter and height of 32 cm and 60 cm, respectively. Each pot had evenly distributed drainage holes at the bottom and a tray below them. It was filled based on a soil bulk density of 1.35 g cm−3, with 54 kg of soil per pot. Each pot was sown with 5–8 cotton seeds, one of which was retained at the three-leaf stage of cotton. Each treatment (CK, M, O, and MO) was conducted in triplicates. The application rates of N, P2O5, and K2O fertilizers in each pot were 10.8 g, 3.6 g, and 3.6 g, respectively. Nitrogen fertilizer was used as the base fertilizer, primary flowering fertilizer, and full flowering fertilizer in a ratio of 3:4:3, respectively, with P and K fertilizers being applied as base fertilizer. During the cotton growth, the soil water content was monitored by the weighing method (Kadayifci et al. 2005), with the water being replenished regularly to maintain the soil moisture content at 60–70% of the field capacity. The same amount of irrigation was used in each treatment.
2.3 Measured Indices
2.3.1 Soil Physical and Chemical Properties
The rhizosphere soil was collected using the shaking-off method (Chiang et al. 2006). The soil pH was measured using a glass electrode after standing for 30 min. The water-soil ratio was 2.5:1, and the soil pH. A DDS-307 conductivity meter (Leica, China) was used to determine the conductivity of soil extract with a water-soil mass ratio of 5:1, and the soil salinity was subsequently obtained by calculation and conversion. The soil organic matter (SOM) was determined using the K dichromate volumetric method (Lu 2000). The TN was determined using the Kjeldahl method after H2SO4 digestion (Bremner 1996). The soil nitrate N (NO3−-N) content was determined by the potassium chloride extraction along with an automatic discontinuous chemical analyzer (Smartchem450, AMS Alliance, Frépillon, France) (Qu et al. 2020). The available P (AP) was determined by the Olsen-P method, while the molybdenum antimony was determined by colorimetry (Bray and Kurtz 1945). The available K (AK) was determined by the ammonium acetate extraction method and atomic absorption spectrophotometer (Z-2000) (Yuan et al. 2011).
2.3.2 Rhizosphere Soil Microorganisms
Soil DNA Extraction, PCR Amplification, and IonS5TMXL Sequencing
The microbial genomic DNA was extracted from the soil samples using a FastDNA SPIN Kit for Soil (MP Biomedicals, Santa Ana, CA, USA). The concentration and purity of the extracted DNA were determined using the Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, USA). The amplification sequence was the V4 region fragment of the 16S rRNA sequence of bacteria, and its primers were 515F (5′-GTGCCAGCMGCCGCGG-3′) and 806R (5′-GGACTACNVGGGTWTCTAAT-3′) (Caporaso et al. 2012). The rRNA and specific gene fragments were amplified by PCR using the Q5 high-fidelity DNA polymerase (NEB, Ipswich, MA, USA). The amplification conditions were 94℃ for 2 min (94℃ for 20 s, 55℃ for 30 s, and 72℃ for 60 s, for 25 cycles), and the final extension step was 72℃ for 10 min. The PCR amplification products were detected via 2.0% agarose gel electrophoresis and purified using a gelation recovery kit by Axygen. The quantitative instruments (microplate reader, FLx800; BioTek, Winooski, Vermont, USA) combined with a fluorescent agent (Quant-iT PicoGreen dsDNA Assay Kit) were used to perform fluorescence quantification of the PCR amplification and recovery products. The sequencing library was prepared using a TruSeq Nano NDA Library Prep Kit (Illumina, San Diego, CA, USA), and the amplified products were subjected to operations. The sequences obtained were divided into operational taxonomic units (OTUs), with the diversity level of samples being evaluated according to the diversity analysis of the OTUs. Based on the analyses of classification composition and statistics, the statistical differences among the groups and the differences in the bacterial community structure among the different samples and species were tested.
Processing the Sequencing Data
The raw sequencing data were processed as described previously (Caporaso et al. 2010). The adaptor and primer sequences were analyzed and quality-filtered using QIIME (Version 1.9.1, http://www.qiime.org). Only sequences > 200 bp with an average quality score > 20 and without ambiguous base calls were included in subsequent analyses. The chimeric sequences were identified and removed using the UCHIME software (http://drive5.com/uchime/). The OTUs with a 97% similarity cutoff were clustered using the UPARSE software (http://drive5.com/uparse/). The representative sequence of each OTU was taxonomically classified by the ribosomal database project (RDP) classifier (http://rdp.cme.msu.edu/) against the SILVA (SSU123) database (https://www.arb-silva.de/) for bacteria and the UNITE database (https://unite.ut.ee/) for fungi using a confidence threshold of 70% (Amato et al. 2013).
Subsampling was conducted by normalizing all the sequence numbers to the minimal number obtained from the treatments by Mothur (Kozich et al. 2013). The relative abundances were determined by the number of sequences affiliated with the same phylogenetic groups divided by the total number of the target phyla or genera per sample (Tian et al. 2015). The rarefaction curves for the bacteria demonstrated that our sequencing data represented most of their compositions (Supplementary Fig. S1). Additionally, the Shannon, Chao1, ACE, and Good’s nonparametric coverage estimator indices were calculated based on Mothur (v.1.25.1, http://www.mothur.org/) to describe the α-diversities for each sample. The sequencing data are deposited in the NCBI database with the accession number SRP342781.
2.4 Statistical Analysis
All the statistical analyses were performed using SPSS version 20.0 (IBM, Inc., Armonk, NY, USA). All the experimental data represented the average of three replicates. Significant differences between the different brackish water activation treatments were determined using the least significant difference (LSD) test and set at the 0.05 probability level. A principal coordinate analysis (PCoA) based on the weighted UniFrac distance was conducted to compare and visualize the similarities among the soil samples using the R vegan package (Oksanen et al. 2007). A heatmap analysis of the top 50 OTUs in four treatments using the Manhattan distance method was performed in the heatmap package (Kolde 2012). The relationships between the soil properties and bacterial communities were analyzed by performing Spearman’s rank correlation analysis and redundancy analysis (RDA) using the R vegan package (Oksanen et al. 2013). All the statistical analyses were conducted using the R software package v.3.5.1.
Networks were used to explore the co-occurrence patterns of the bacterial taxa. The molecular ecological network analysis pipeline (MENAP) between bacteria was constructed using the random matrix theory (RMT)-based network approach (Ye et al. 2012). The MENAP construction and analyses were performed on http://ieg4.rccc.ou.edu/MENA/main.cgi. The OTUs detected in over half of the sampling sites were kept for constructing the network. The missing values were filled through the nearest neighbor method (Olga et al. 2001), which calculated the mean of the remaining values for the missing positions. The topological characteristics of co-occurring networks were calculated to describe the interspecies interaction of bacterial OTUs: modularity (MD), average connectivity (avgK), average clustering coefficient (avgCC), and average path length (avgPL). A module is a group of OTUs that is well connected among its members but is less linked with OTUs belonging to other modules (Olesen et al. 2007). The role of each OTU was determined based on its position as compared with other OTUs in its module, and how well it connected to nodes in the other modules. Therefore, the role of OTU in the network was characterized by its within-module connectivity (Zi) and among-the-module connectivity (Pi) (Guimerà and Amaral 2005). According to the simplified criteria, all the species were sorted into four subcategories: (1) peripherals, (2) connectors, (3) module hubs, and (4) network hubs (Olesen et al. 2007). Networks were explored and visualized using the Cytoscape (2.6.0) software (Cline et al. 2007). Additionally, random networks with an equal number of nodes and edges as the experimental networks were generated based on the Erdös-Réyni model (Erdös and Réyni 1960). The topological properties of the random network were calculated using the average from 10,000 Erdös-Réyni random networks using the igraph and plyr packages in the R environment. The theoretical incidence of co-occurrence was calculated by considering the taxa frequencies and assuming random association (Barberán et al. 2012). The degree of disagreement between the experimental and random co-occurring incidences was used to explore the nonrandom assembly patterns in the bacterial communities (Ju et al. 2014).
3 Results and Discussion
3.1 Effect of Irrigation with Activated Brackish Water on the Physical and Chemical Indices of Rhizosphere Soil
Compared with the CK treatment, irrigation with magnetized or oxygenated brackish water reduced the salt accumulation around the rhizosphere soil (Table 2), which was consistent with previous studies (Bhattarai et al. 2005; Radhakrishnan and Ranjitha 2012; Zhou et al. 2022). This is because, after the magnetization or oxygenation treatment, the associated water molecule cluster structure of the irrigation water was dispersed into free monomers and dimers, thereby increasing the likelihood of water molecules entering the tiny soil pores. The movement of salt in the soil follows the migration law of “salt moves with water” (Zhou et al. 2021a, b). Therefore, increasing water entering the soil pores could wash away more salt, thereby reducing the soil salinity. We showed that the MO treatment was the most effective in reducing the soil salt content. Additionally, there were no significant differences in the soil pH and TN content among the different treatments. However, as compared with CK, the M, O, and MO treatments reduced the contents of SOM, NO3−-N, AP, and AK in the rhizosphere soil, with the difference between MO treatment and CK treatment being the most significant (Table 2). However, the main reason is that the activated brackish water irrigation was better at improving the soil environment and increasing its nutrient availability, than brackish water irrigation. For example, Abuarab et al. (2013) and Bhattarai et al. (2009) showed that irrigation with oxygenated water could significantly increase the rate of soil respiration, thereby accelerating the soil organic matter decomposition and the release of its nutrients. Noran et al. (1996) proposed that magnetic treatment of water might make crops release more organic acids in the rhizosphere, which then would affect the desorption of P and K adsorbed on the soil colloidal complexes, thereby increasing the effectiveness and bioavailability of these nutrients for crops. Contrastingly, magnetized or oxygenated brackish water irrigation could improve the root activity and promote the efficient absorption of the soil available nutrients by the roots (Radhakrishnan and Ranjitha 2012; Zhao et al. 2022). Particularly, during the vegetative and reproductive growth periods of cotton, the transformation and absorption efficiency of the soil nutrient was higher, whereas the intensity of soil nutrient supply decreased during the cotton harvest period. Through the metagenomic data of soil microorganisms in our early study (data unpublished), we also found that the relative abundance of the soil functional microorganisms decreased, and their metabolic functions were also weakened after the reproductive and growth period of crops. Therefore, the above statements further showed that activation treatment may affect the soil’s nutrient transport capacity by changing the physicochemical properties of irrigation water (Table 1). For example, the surface tension reduction weakened the ability of water to rise in the capillary, thereby improving the ability of water molecules to transport nutrients in the soil. Additionally, increasing the concentration of dissolved oxygen in irrigation water favored the transformation of soil nutrients, thereby improving their ability to be supplied. Among them, the effect of MO treatment on the soil desalination and nutrient content was the most significant.
3.2 Analysis of Bacterial Diversity in the Rhizosphere Soil Following Irrigation with Activated Brackish Water
3.2.1 Analysis of Bacterial Community Diversity
We observed no significant difference in the bacterial community diversity between the irrigation with brackish water and activated brackish water (Table 3). Our results showed that the brackish water did not significantly affect the OTU number (species number) and bacterial community diversity post activation treatment. This was inconsistent with the results of Cui et al. (2020), where magnetized brackish water irrigation significantly improved the bacterial diversity indices. This may be because, in this study, since the soil was slightly saline-alkali soil, the salt accumulation in the soil under brackish water irrigation did not significantly affect the bacterial diversity indices. Furthermore, it may also be related to the insignificant effect of activated brackish water irrigation on the soil bacterial diversity-sensitive factors (such as pH, Table 2) (Xiao et al. 2021). Additionally, the activated brackish water irrigation improved the soil fertility, which may confer the dominant populations in the microflora a strong ability to resist external interference to the soil environment (Irsad et al. 2022), resulting in no significant impact on the soil bacterial diversity.
3.2.2 Correlation Analysis Between the Soil Bacterial Diversity and the Physical and Chemical Properties of Soil
A Spearman correlation analysis showed that there was no significant correlation between the bacterial diversity index and most of the soil’s physical and chemical indices (Table 4). However, there was a very significant negative correlation between the soil TN content and the Chao1 index. This indicated that the increase in the soil TN content could significantly inhibit the soil bacterial community richness. Soil TN is an important indicator to evaluate soil fertility and soil quality (Bahram et al. 2018; Kuypers et al. 2018). Numerous studies have found that the N enrichment of soil usually causes the loss of the local soil microbial diversity (Xiao et al. 2018; Yang et al. 2020; Zhou et al. 2017). This is mainly because the N enrichment frees soil microorganisms from the N limitation, allowing them to strongly compete for other limited natural resources (Tripathi et al. 2018). For example, under the condition of high TN content in the soil, some ammonium- and acid-loving microbial species would face stiff competition with each other and become extinct in large numbers, thereby reducing the soil bacterial diversity (Lundberg and Teixeira 2018). Therefore, the above results showed that under brackish water irrigation, the activation treatment was not the driving factor affecting the soil bacterial diversity, but the increase of soil TN content inhibited the soil bacterial diversity.
3.2.3 Analysis of the Bacterial Community Structure in Rhizosphere Soil Following Activation with Brackish Water
Analysis of Bacterial Community Composition
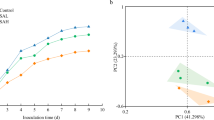
PCoA showed that there were significant differences in the soil bacterial community structure post irrigation with differently treated activated brackish water (Fig. 1). Among them, the O and MO treatments differed significantly from those of CK and M treatments. This indicated that as compared with the treatment of single magnetization, the irrigation with oxygenated brackish water and magnetized-oxygenated brackish water were more likely to increase the relative abundance of microorganisms in some dominant populations in the soil.
Under different activated brackish water irrigation conditions, the composition of soil bacteria was similar at the phylum level, but the relative abundance of some bacterial phyla differed significantly (Fig. 2). Proteobacteria, Chloroflexi, Actinobacteria, Gemmatimonadetes, and Acidobacteria were the five phyla with the highest bacterial abundance in all the soils, comprising 81.6% of the total bacterial sequences. Furthermore, it was also proven that these bacteria are ubiquitous in the saline-alkali soil (Li et al. 2016). However, the relative abundance of Chloroflexi in the O treatment was significantly higher than that in the CK treatment. During its role in the biogeochemical cycle of N and carbon (C), Chloroflexi requires oxygen, thereby making its relative abundance sensitive to oxygen concentration (Islam et al. 2019). The relative abundance of Actinobacteria post the MO treatment was significantly higher than that of the O treatment, whereas that of Gemmatimonadetes post the MO treatment was significantly lower than those of the other three treatments. Actinobacteria in soil accelerate the decomposition of animal and plant residues and also have some role in the C and N cycles (Lauber et al. 2008). Many members of Gemmatimonadetes are actively involved in the process of biogeochemical transformation, particularly in highly saline soil (Zhang et al. 2003). It was found that the relative abundance of Gemmatimonadetes in highly saline soil was significantly higher than that in soil with low-level salinity (Canfora et al. 2014). The relative abundance of Acidobacteria post the O and MO treatments were significantly higher than that of the CK and M treatments. Acidobacteria prefer a weakly acidic environment (Michelle et al. 2006) and participate in the soil’s C and N cycles (Ward et al. 2009; Ye et al. 2012). Additionally, Acidobacteria has been found to increase under low salinity conditions and decrease under high salinity conditions (Zheng et al. 2017). Therefore, these results indicate that irrigation with oxygenated brackish water reduced the salinity of the rhizosphere soil, with the MO treatment supporting the soil C and N cycles more than the simple O treatment.
Based on the cluster analysis, the bacterial relative abundance of MO treatment at the genus level significantly differed from those of the other three treatments, with the O treatment significantly differing from the CK and M treatments (Fig. 3). This indicated that different activation treatments of brackish water significantly affected the relative abundance of dominant bacterial genera in the soil. The dominant bacterial genera of each treatment were significantly different (Table 5). For example, the bacterial relative abundance (primarily including Gemmatimonas and Enhygromyxa) in the CK treatment was significantly higher than in the other treatments. Gemmatimonas participate in the soil’s C and P cycle and may suppress disease and promote plant growth (Li et al. 2017). Enhygromyxa is a halophilic Myxobacterium with strong salinity tolerance (Iizuka et al. 2013). The bacterial relative abundance in the O treatment primarily included Opitutus, Nitrospira, and Pelagibius. Opitutus reduces the soil nitrate content by reducing nitrate to nitrite (Liu et al. 2021). Nitrospira, as a nitrifier, oxidizes nitrite to nitrate and has a high salinity tolerance (Daebeler et al. 2020). Pelagibius can inhibit urease and alkaline phosphatase activities, which prevent the circulation of soil N and P (Li et al. 2018a, b). The bacterial relative abundance (primarily including Salinirepens and AKYG587) in the M treatment was significantly higher than those in the other treatments. Saliniprepens has a strong salt tolerance (Muramatsu et al. 2012). AKYG587 is mainly found in dry soil and belongs to heterotrophic denitrifying bacteria, which can reduce the soil’s NO3−-N availability (Delmont et al. 2018). The bacterial relative abundance (primarily including Blastococcus and Azoarcus) in the MO treatment was significantly higher than those in the other treatments. Blastococcus showed strong resistance to oxidative stress (Gtari et al. 2012), while Azoarcus needs oxygen for respiration and metabolism and also fixes N (Chen et al. 2013). Therefore, these results indicated that brackish water irrigation increased the relative abundance of salt-tolerant bacteria in the soil, while activated brackish water irrigation affected the number of soil functional bacteria. For example, either a single O or M treatment reduced the soil nutrient (e.g., N and P) availability, while the MO treatment mitigated the effect of oxidative stress and facilitated the function of functional bacteria (e.g., N fixation) in the soil. This is because, under salt stress (brackish water irrigation), the O or M treatment increases the dissolved oxygen content of the irrigation water. So, too high or too low dissolved oxygen content will affect the soil redox potential and subsequently inhibited the enzyme activity that transforms the soil nutrients (Merlo et al. 2014). The MO treatment can maintain a suitable redox potential, which favors the improvement of soil enzyme activity and the transformation of soil C and N. Additionally, Bacillus, Candidatus, Solibacter, Gaiella, H16, and Haliangium did not differ significantly in the four treatments.
Correlation Analysis Between the Soil Bacterial Flora and Physical and Chemical Properties of the Soil
In this study, the correlation between the soil bacterial flora and soil environmental factors was illustrated by an RDA analysis (Fig. 4, Table 6). The soil bacterial flora and environmental variables are represented by arrows, and the different activation treatments of brackish water are represented by symbols of different colors. Soil physical and chemical properties comprised 51.28% of the variation in soil bacterial flora. Soil organic matter, NO3−-N, and pH were closely related to the bacterial community structure in saline-alkaline soil. These soil indices have been proven to be important factors affecting the bacterial community composition in saline-alkaline soils (Banerjee et al. 2016; Shen et al. 2016). Since the brackish water irrigation during the entire growth period of cotton increased the soil salt content, the rhizosphere soil salinity was the main factor affecting the bacterial community composition in the CK treatment. The O and M treatments were related to Gemmatimonadetes and Planctomycetes, with these bacterial phyla showing a significantly negative correlation with the soil TN and NO3−-N (p < 0.05). Both Gemmatimonadetes and Planctomycetes are important in the soil N cycle (Guo et al. 2015; Li and Han 2015). Moreover, Acidobacteria and Actinobacteria primarily existed in the MO treated rhizosphere soil and were primarily dominated by soil nutrient indices. Among them, Actinobacteria showed a significantly positive correlation with the SOM and NO3−-N (p < 0.05), which was consistent with the findings of Liu et al. (2014). Additionally, Acidobacteria showed a significantly positive correlation with the soil AP (p < 0.05). A previous study showed that the high soil P concentration could be a potential determinant of Acidobacteria activity, and it would affect the relative abundance of Acidobacteria (Foesel et al. 2014).
A redundancy analysis (RDA) reflects the relationship between soil characteristics and bacterial communities. CK, brackish water; M, magnetized brackish water; O, oxygenated brackish water; MO, magnetized-oxygenated brackish water. SOM, soil organic matter; TN, total N; NO3−-N, nitrate N; AP, available P; AK, available K
Soil pH and salinity were equally important in constructing the bacterial communities in saline-alkaline soils (Zhao et al. 2018a, b). Interestingly, the salinity and soil nutrients played a relatively more important role in shaping the bacterial community distribution than pH in our study. Therefore, we infer that the bacterial communities might be more sensitive to salinity and nutrient availability, possibly because our study only included alkaline soils with a small pH range, and the pH values of different activated irrigation water were significantly not different (Table 1). Additionally, the soil pH value at harvest was not significantly different from that before planting. Therefore, under the condition of activated brackish water irrigation, the soil salinity and nutrient availability were the main driving factors affecting the bacterial community in the saline-alkaline soil.
3.2.4 Distribution and Symbiosis of Bacteria Following Irrigation with Activated Brackish Water
Herein, we selected the bacterial OTUs that had a relative abundance > 0.01% for ecological network analysis based on the molecular ecological network analysis standard process (Ye et al. 2012). After screening the original OTUs, 340 OTUs of the different activated brackish water treatments met the network construction requirements (Table 7). We constructed the microbial community ecological network using the random matrix theory (Fig. 5), in which each colored dot represents a node (OTU); each line represents a correlation between the two connected nodes; the red line represents a positive correlation between the two nodes, and the blue line represents a negative correlation between the two nodes. There were 732 links in the microbial network, of which 560 and 172 were positively and negatively correlated, respectively, thereby indicating that most soil bacteria exhibited a cooperative relationship. There were 15 complex modules with over four nodes in the bacterial network module.
The bacterial network comprised a series of OTUs, with each OTU playing a different role in the network topology. In this study, it was represented by a Zi-Pi scatter diagram (Fig. 6). Based on the Zi and Pi scatter diagrams, it could be divided into four quadrants that represent four types of nodes: (1) The nodes in quadrants of Zi < 2.5 and 0 ≤ Pi < 0.62 were “peripherals” (specialties), which had little contact with the outside world and were almost only related to the nodes in their module. (2) The nodes in the quadrant of Zi ≥ 2.5 and Pi < 0.62 were “module” hubs (generalists), which were closely related to the nodes in their respective modules. (3) The nodes in the quadrant of Zi < 2.5 and Pi ≥ 0.62 were “connectors” (generalists), which were closely related to the modules. (4) The nodes in the quadrant of Zi ≥ 2.5 and Pi ≥ 0.62 were “network” hubs (super generalists), which were closely related to both modules and nodes in the modules, thereby playing the dual role of “connector” and “module” hubs. In the bacterial network of this study, 327 nodes (comprising 96.2% of the total nodes) were “peripherals” (specialists); eight nodes (comprising 2.3% of the total nodes) were “connectors” (generalists); and five nodes (comprising 1.5% of the total nodes) were members of “module” hubs (Generalists). The classification information of “connectors” (generalists) and “module” hubs (generalists) corresponding to specific OTUs is shown in Fig. 6. Among them, the “module” hubs (generalists) comprised Gemmatimonadetes, Betaproteobacteria, Gemmatimonadaceae, Nitrosomonadacea, and Coxiella_endosymbiont_of_Ornithodoros_marocanus, all of which were members of the Gemmatimonadetes and Proteobacteria phyla. “Connectors” (generalists) comprised Chloroflexi, Gemmatimonadetes, Bacteroidetes, Thermomicrobia, Rhizobiales, H16, Sulfurifustis, and Woodsholea, all of which were members of the Chloroflexi, Gemmatimonadetes, Bacteroidetes, and Proteobacteria phyla.
Zi-Pi plot showing the distribution of bacterial OTUs based on their topological roles following treatment with different types of activated brackish water. Each filled circle represents an OTU in the bacterial network. The threshold values of Zi and Pi for categorizing the OTUs were 2.5 and 0.62, respectively
The experimental network structural indices constructed under the similarity threshold (0.880) differed significantly (p < 0.05) from those in the random network. The avgCC, avgPL, and MD of the experimental network were higher than those of the random network. Higher avgK values indicated that the network was more complex, with those networks having a lower avgPL value showing a closer connection among the nodes (Ye et al. 2012). A higher MD value of the network indicated a higher community organization order. The MD index in this study was greater than the recommended threshold (0.4) (Newman 2006), thus indicating that the network had better modularity, and the bacterial network following irrigation with activated brackish water was scale-free, small world, and modular (Clauset et al. 2008). Similar to a human social network, the existence of more interconnected individuals in the network means greater cooperation and communication, consequently indicating the network can operate more efficiently and achieve common goals more easily. Therefore, the Zi-Pi scatter diagram analysis indicated that the number of “generalists” was vital to the network. Without “generalists,” the nodes within or between modules would be unable to communicate with the other nodes, causing a disordered network that was unable to carry out effective organization and exchange of energy, materials, and information, ultimately hampering its ability to operate effectively. The relative abundance of “connector” and “module” hubs in different treatments is shown in Table 8. Betaproteobacteria, Gemmatimonadaceae, Nitrosomonadaceae, Sulfurifustis, and H16 did not differ significantly (p > 0.05) among the four treatments.
Compared with irrigation using brackish water, the O treatment significantly increased the relative abundance of Chloroflexi, Thermomicrobia, and Coxiella_endosymbiont_of_Ornithodoros_marocanus. Chloroflexi generated energy via photosynthesis, but their ecological function still remained unclear (Fullerton and Moyer 2016; Shao et al. 2020). The Coxiella_endosymbiont_of_Ornithodoros_marocanus is a member of Coxiella genus, which is a soil-borne pathogen (Suyal et al. 2021). Irrigation with magnetized brackish water significantly increased the relative abundance of the Coxiella_endosymbiont_of_Ornithodoros_marocanus. The MO treatment significantly increased the relative abundance of Thermomicrobia, while significantly reducing that of Coxiella_endosymbiont_of_Ornithodoros_marocanus. Some studies showed that most Thermomicrobia were thermophilic bacteria and were closely related to N fixation function (Morales et al. 2010). Therefore, compared with brackish water irrigation, activated brackish water had a more significant effect on the soil bacterial community structure. Furthermore, during activated brackish water irrigation, the MO treatment was not only more beneficial in reducing the colonization of soil-borne pathogens but also promoted the circulation and transformation of soil nutrients when compared with single M or O treatment.
4 Conclusions
The activated brackish water irrigation was more effective than brackish water irrigation in reshaping the soil bacterial community composition. Compared with irrigation using brackish water, using activated brackish water reduced not only the salt accumulation in the rhizosphere soil but also the content of NO3−-N, AP, and AK during cotton maturity. There were significant differences in the soil bacterial community structure post irrigation with different types of activated brackish water. The study of microbial distribution and symbiosis law revealed that the rhizosphere soil irrigated with oxygenated brackish water and magnetized brackish water favored the reproduction of some soil-borne pathogens and did not support the crop root growth. The MO treatment significantly reduced the colonization of soil-borne pathogens in the rhizosphere soil, while also favoring the function of functional bacteria involved in soil nutrient transformation. Therefore, the MO treatment is the most suitable activation mode for cotton planting in saline-alkaline soil under brackish water irrigation.
References
Abd Elhady SA, Abd El-Gawad HG, Ibrahim MFM, Mukherjee S, Elkelish A, Azab E, Gobouri AA, Farag R, Ibrahim HA, Ibrahim HA (2021) Hydrogen peroxide supplementation in irrigation water alleviates drought stress and boosts growth and productivity of potato plants. Sustainability 13(2):899. https://doi.org/10.3390/su13020899
Abuarab M, Mostafa E, Ibrahim M (2013) Effect of air injection under subsurface drip irrigation on yield and water use efficiency of corn in a sandy clay loam soil. J Adv Res 4(6):493–499. https://doi.org/10.1016/j.jare.2012.08.009
Aivazidou E, Tsolakis N (2021) Investigating dynamic interconnections between organic farming adoption and freshwater sustainability. J Environ Manage 294:112896. https://doi.org/10.1016/j.jenvman.2021.112896
Al-Ogaidi AAM, Wayayok A, Rowshon MK, Abdullah AF (2017) The influence of magnetized water on soil water dynamics under drip irrigation systems. Agr Water Manage 180:70–77. https://doi.org/10.1016/j.agwat.2016.11.001
Amato KR et al (2013) Habitat degradation impacts black howler monkey (alouatta pigra) gastrointestinal microbiomes. ISME J 7:1344–1353. https://doi.org/10.1038/ismej.2013.16
Bahram M, Hildebrand F, Forslund SK, Anderson JL, Soudzilovskaia NA, Bodegom PM (2018) Structure and function of the global topsoil microbiome. Nature 560:233–237. https://doi.org/10.1038/s41586-018-0386-6
Banerjee S, Helgason B, Wang L, Winsley T, Ferrari BC, Siciliano SD (2016) Legacy effects of soil moisture on microbial community structure and N2O emissions. Soil Biol Biochem 95:40–50. https://doi.org/10.1016/j.soilbio.2015.12.004
Barberán A, Bates ST, Casamayor EO, Fierer N (2012) Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J 6:343–351. https://doi.org/10.1038/ismej.2011.119
Bhattarai SP, Su N, Midmore DJ (2005) Oxygation unlocks yield potentials of crops in oxygen-limited soil environments. Adv Agron 88:313–377. https://doi.org/10.1016/S0065-2113(05)88008-3
Bhattarai SP, Midmore DJ, Pendergast LP (2008) Yield, water-use efficiencies and root distribution of soybean, chickpea and pumpkin under different subsurface drip irrigation depths and oxygation treatments in vertisols. Irrigation Sci 26:439–450. https://doi.org/10.1007/s00271-008-0112-5
Bhattarai SP, Pendergast L, Midmore DJ (2009) Oxygation enhances growth, gas exchange and salt tolerance of vegetable soybean and cotton in a saline vertisol. J Integr Plant Biol 51(7):675–688 (CNKI:SUN:ZWXB.0.2009-07-007)
Bray RH, Kurtz LT (1945) Determination of total, organic, and available forms of phosphorus in soils. Soil Sci 59:39–46. https://doi.org/10.1097/00010694-194501000-00006
Bremner JM (1996) Total nitrogen. In: Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnson CT, Sumner ME. (Eds.), Methods of Soil Analysis, Part 3. Chemical Methods, pp. 1085–1122 Madison, Wisconsin, USA.
Canfora L, Bacci G, Pinzari F, Lo PG, Dazzi C, Benedetti A (2014) Salinity and bacterial diversity: to what extent does the concentration of salt affect the bacterial community in a saline soil? PLoS ONE 9:1–15. https://doi.org/10.1371/journal.pone.0106662
Capone DG, Knapp AN (2007) Oceanography-A marine nitrogen cycle fix? Nature 445:159–160. https://doi.org/10.1038/445159a
Caporaso JG et al (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. https://doi.org/10.1038/nmeth.f.303
Caporaso JG et al (2012) Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6:1621–1624. https://doi.org/10.1038/ismej.2012.8
Chen MH, Sheu SY, James EK, Young CC, Chen WM (2013) Azoarcus olearius sp. nov., a nitrogen-fixing bacterium isolated from oil-contaminated soil. Int J Syst Evol Micr 63:3755–3761. https://doi.org/10.1099/ijs.0.050609-0
Cheng MH, Wang HD, Fan JL, Wang XK, Sun X, Yang L, Zhang SH, Xiang YZ, Zhang FC (2021) Crop yield and water productivity under salty water irrigation: a global meta-analysis. Agr Water Manage 256:107105. https://doi.org/10.1016/j.agwat.2021.107105
Chiang PN, Wang MK, Chiu CY, Chou SY (2006) Effects of cadmium amendments on low-molecular-weight organic acid exudates in rhizosphere soils of tobacco and sunflower. Environ Toxicol 21(5):479–488. https://doi.org/10.1002/tox.20210
Clauset A, Moore C, Newman ME (2008) Hierarchical structure and the prediction of missing links in networks. Nature 453:98–101. https://doi.org/10.1038/nature06830
Cline MS, Smoot M, Cerami E, Kuchinsky A, Landys N, Workman C, Christmas R, Avilacampilo I, Creech M, Gross B (2007) Integration of biological networks and gene expression data using Cytoscape. Nat Protoc 2:2366–2382. https://doi.org/10.1038/nprot.2007.324
Cui HR, Liu XM, Jing RY, Zhang MZ, Wang L, Zheng L, Kong LG, Wang HT, Ma FY (2020) Irrigation with magnetized water affects the soil microenvironment and fruit quality of eggplants in a covered vegetable production system in Shouguang city. China J Soil Sci Plant Nut 20(4):2684–2697. https://doi.org/10.1007/s42729-020-00334-7
Daebeler A et al (2020) Exploring the upper pH limits of nitrite oxidation: diversity, ecophysiology, and adaptive traits of haloalkalitolerantNitrospira. ISME J 14(12):2967–2979. https://doi.org/10.1038/s41396-020-0724-1
Dedysh SN, Kulichevskaya IS, Beletsky AV, Ivanova AA, Rijpstra WIC, Damste JSS, Mardanov AV, Ravin NV (2020) Lacipirellula parvula gen. nov., sp. nov., representing a lineage of planctomycetes widespread in low-oxygen habitats, description of the family Lacipirellulaceae fam. nov. and proposal of the orders Pirellulales ord. nov., Gemmatales ord. nov. and Isosphaerales ord. nov. Syst Appl Microbiol 43(1):126050. https://doi.org/10.1016/j.syapm.2019.126050
Delmont TO, Quince C, Shaiber A, Esen ÖC, Lee STM, Rappé MS, MacLellan SL, Lücker S, Eren AM (2018) Nitrogen-fixing populations of planctomycetes and proteobacteria are abundant in surface ocean metagenomes. Nat Microbiol 3(7):804–813. https://doi.org/10.1038/s41564-018-0176-9
Erdös P, Réyni A (1960) On the evolution of random graphs. Publ Math Inst Hung Acad Sci 5:17–61. https://doi.org/10.1515/9781400841356.38
Fan J, Zhang B, Zhang J, Ngo HH, Wu HM (2013) Intermittent aeration strategy to enhance organics and nitrogen removal in subsurface flow constructed wetlands. Bioresource Technol 141:117–122. https://doi.org/10.1016/j.biortech.2013.03.077
Foesel BU et al (2014) Determinants of Acidobacteria activity inferred from the relative abundances of 16S rRNA transcripts in German grassland and forest soils. Environ Microbiol 16(3):658–675. https://doi.org/10.1111/1462-2920.12162
Fu CP, Wu JF, Han JY, Zhao L, Chan G, Leong KF (2020) Effects of substrate type on denitrification efficiency and microbial community structure in constructed wetlands. Bioresource Technol 307:123222. https://doi.org/10.1016/j.biortech.2020.123222
Fullerton H, Moyer CL (2016) Comparative single-cell genomics of chloroflexi from the okinawa trough deep-subsurface biosphere. Appl Environ Microb 82:3000–3008. https://doi.org/10.1128/AEM.00624-16
Gtari M et al (2012) Contrasted resistance of stone-dwelling Geodermatophilaceae species to stresses known to give rise to reactive oxygen species. FEMS Microbiol Ecol 80:566–577. https://doi.org/10.1111/j.1574-6941.2012.01320.x
Guimerà R, Amaral L (2005) Cartography of complex networks: modules and universal roles. J Stat Mech P02001:1–13. https://doi.org/10.1088/1742-5468/2005/02/P02001
Guo Y, Gong H, Guo X (2015) Rhizosphere bacterial community of Typha angustifolia L. and water quality in a river wetland supplied with reclaimed water. Appl Microbiol Biotechnol 99:2883–2893. https://doi.org/10.1007/s00253-014-6182-9
Hicks LC, Rousk K, Rinnan R, Rousk J (2020) Soil microbial responses to 28 years of nutrient fertilization in a subarctic heath. Ecosystems 23:1107–1119. https://doi.org/10.1007/s10021-019-00458-7
Hou J, Zhang DY, Zhou JL, Xu Z, Mou P, Cao YX, Zhu JQ (2021) Oxygenated compound fertilizer improved rice yield by empowering soil aeration. Commun Soil Sci Plan 52(15):1798–1810. https://doi.org/10.1080/00103624.2021.1900219
Hozayn M, Abdallha MM, Monem AEI, El Saady AA, Darwish MA (2016) Applications of magnetic technology in agriculture: a novel tool for improving crop productivity (1): Canola. Afr J Agr Res 11:441–449. https://doi.org/10.5897/AJAR2015.9382
Hozayn MM, Salim MA, Abd El-Monem AA, El-Mahdy AA (2019) Effect of magnetic brackish water treatments on morphology, anatomy and yield productivity of wheat (Triticum aestivum). Alex Sci Exch J 40:604–617. https://doi.org/10.21608/asejaiqjsae.2019.63578
Iizuka T, Jojima Y, Hayakawa A, Fujii T, Yamanaka S, Fudou R (2013) Pseudenhygromyxa salsuginis gen. nov., sp nov., a myxobacterium isolated from an estuarine marsh. Int J Syst Evol Micr 63:1360–1369. https://doi.org/10.1099/ijs.0.040501-0
Irsad ASK, Talreja N, Chauhan D, Rizvi PQ, Ashfaq M (2022) Current status, future challenges, and opportunities for improving the crop yields using microorganisms. Recent Adv Food Biotechnol 9:175–192. https://doi.org/10.1007/978-981-16-8125-7_9
Islam ZF et al (2019) Two Chloroflexi classes independently evolved the ability to persist on atmospheric hydrogen and carbon monoxide. ISME J 13(7):1801–1813. https://doi.org/10.1038/s41396-019-0393-0
Jiang SY et al (2021) NIN-like protein transcription factors regulate leghemoglobin genes in legume nodules. Science 374(6567):625–628. https://doi.org/10.1126/science.abg5945
Ju F, Xia Y, Guo F, Wang ZP, Zhang T (2014) Taxonomic relatedness shapes bacterial assembly in activated sludge of globally distributed wastewater treatment plants. Environ Microbiol 16:2421–2432. https://doi.org/10.1111/1462-2920.12355
Kadayifci A, Tuylu GI, Ucar Y, Cakmak B (2005) Crop water use of onion (Allium cepa L) in Turkey. Agr Water Manage 72(1):59–68. https://doi.org/10.1016/j.agwat.2004.08.002
Ke X, Lu W, Conrad R (2015) High oxygen concentration increases the abundance and activity of bacterial rather than archaeal nitrifiers in rice field soil. Microb Ecol 70(4):961–970. https://doi.org/10.1007/s00248-015-0633-4
Kolde R (2012) Pheatmap: Pretty Heatmaps. R Package Version 61:617. https://doi.org/10.1371/journal.pone.0052730
Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD (2013) Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 79:5112–5120. https://doi.org/10.1128/AEM.01043-13
Kuypers MM, Marchant HK, Kartal B (2018) The microbial nitrogen-cycling network. Nat Rev Microbiol 16(5):263. https://doi.org/10.1038/nrmicro.2018.9
Lauber CL, Strickland MS, Bradford MA, Fierer N (2008) The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol Biochem 40:2407–2415. https://doi.org/10.1016/j.soilbio.2008.05.021
Li X, Sun M, Zhang H, Xu N, Sun G (2016) Use of mulberry–soybean intercropping in salt–alkali soil impacts the diversity of the soil bacterial community. Microb Biotechnol 9:293–304. https://doi.org/10.1111/1751-7915.12342
Li F, Chen L, Zhang JB, Yin J, Huang SM (2017) Bacterial community structure after long-term organic and inorganic fertilization reveals important associations between soil nutrients and specific taxa involved in nutrient transformations. Front Microbiol 8:187. https://doi.org/10.3389/fmicb.2017.00187
Li M, Fu Q, Singh VP, Ji Y, Liu D, Zhang CL, Li TX (2018a) An optimal modelling approach for managing agricultural water-energy-food nexus under uncertainty. Sci Total Environ 651:1416–1434. https://doi.org/10.1016/j.scitotenv.2018.09.291
Li WH, Liu QZ, Chen P (2018b) Effect of long-term continuous cropping of strawberry on soil bacterial community structure and diversity. J Integr Agr 17(11):2570–2582. CNKI:SUN:ZGNX.0.2018b–11–022
Liu J, Sui Y, Yu Z, Shi Y, Chu H, Jin J, Liu X, Wang G (2014) High throughput sequencing analysis of biogeographical distribution of bacterial communities in the black soils of northeast China. Soil Biol Biochem 70:113–122. https://doi.org/10.1016/j.soilbio.2013.12.014
Liu BX, Wang SQ, Kong XL, Liu XJ, Sun HY (2019a) Modeling and assessing feasibility of long-term brackish water irrigation in vertically homogeneous and heterogeneous cultivated lowland in the North China Plain. Agr Water Manage 211:98–110. https://doi.org/10.1016/j.agwat.2018.09.030
Liu X, Zhu H, Meng S, Bi S, Zhang Y, Wang H, Song C, Ma F (2019b) The effects of magnetic treatment of irrigation water on seedling growth, photosynthetic capacity and nutrient contents of Populus × euramericana ‘Neva’ under NaCl stress. Acta Physiol Plant 41:11. https://doi.org/10.1007/s11738-018-2798-1
Liu S, Wang ZY, Wang ZZ (2021) Changes in physicochemical properties, enzymatic activities, and the microbial community of soil significantly influence the continuous cropping of Panax quinquefolius L (American ginseng). Plant Soil 463(1–2):427–446. https://doi.org/10.1007/s11104-021-04911-2
Lu RK (2000) Chemical analysis method of agricultural soil. China Agricultural Science And Technology Publisher, Beijing
Lundberg DS, Teixeira PJ (2018) Root-exuded coumarin shapes the root microbiome. P Natl A Sci 115:5629–5631. https://doi.org/10.1073/pnas.1805944115
Merlo C, Reyna L, Abril A, Ame MV, Genti-Raimondi S (2014) Environmental factors associated with heterotrophic nitrogen-fixing bacteria in water, sediment, and riparian soil of Suquia River. Limnologica 48:71–79. https://doi.org/10.1016/j.limno.2014.06.004
Mghaiouini R, Salah M, Monkade M, El Bouari A (2021) New green process to increase the solubility of soil fertilizer based on potassium nitrate (KNO3). J Indian Chem Soc 98(12):100224. https://doi.org/10.1016/j.jics.2021.100224
Michelle S, Kathryn ER, Peter H (2006) Effect of pH on isolation and distribution of members of subdivision 1of the phylum Acidobacteria occurring in soil. Appl Environ Microb 72:1852–1857. https://doi.org/10.1128/AEM.72.3.1852-1857.2006
Moosavi F, Gholizadeh M (2014) Magnetic effects on the solvent properties investigated by molecular dynamics simulation. J Magn Magn Mater 354:239–247. https://doi.org/10.1016/j.jmmm.2013.11.012
Morales SE, Cosart T, Holben WE (2010) Bacterial gene abundances as indicators of greenhouse gas emission in soils. ISME J 4(6):799–808. https://doi.org/10.1038/ismej.2010.8
Morejón LP, Palacio JCC, Velázquez AL, Govea AP (2007) Stimulation of pinus tropicalis m. seeds by magnetically treated water. Int Agrophys 21:173–177. https://doi.org/10.1016/j.indcrop.2006.08.001
Muramatsu Y, Takahashi M, Kamakura Y, Suzuki K, Nakagawa Y (2012) Salinirepens amamiensis gen. nov., sp nov., a member of the family Cryomorphaceae isolated from seawater, and emended descriptions of the genera Fluviicola and Wandonia. Int J Syst Evol Micr 62:2235–2240. https://doi.org/10.1099/ijs.0.032029-0
Newman ME (2006) Modularity and community structure in networks. P Natl Acad Sci USA 103:8577–8582. https://doi.org/10.1073/pnas.0601602103
Noran R, Shani R, Lin I (1996) The effect of irrigation with magnetically treated water on the translocation of minerals in the soil. Magn Electr Sep 7 109–122. https://doi.org/10.1155/1996/46596
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2013) Package ‘vegan.’ CommUN Ecol Package R Package Version 2:10. https://doi.org/10.1109/MSPEC.2006.1611759
Oksanen J et al (2018) Vegan: community ecology package. R package version 2.5–2. https://CRAN.R-project.org/package=vegan
Olesen JM, Bascompte J, Dupont YL, Jordano P (2007) The modularity of pollination networks. P Natl Acad Sci USA 104:19891–19896. https://doi.org/10.1073/pnas.0706375104
Olga T, Michael C, Gavin S, Pat B, Trevor H, Robert T, Altman RB (2001) Missing value estimation methods for dna microarrays. Bioinformatics 17:520–525. https://doi.org/10.1093/bioinformatics/17.6.520
Pan J, Fei H, Song S, Yuan F, Yu L (2015) Effects of intermittent aeration on pollutants removal in subsurface wastewater infiltration system. Bioresource Technol 191:327–331. https://doi.org/10.1016/j.biortech.2015.05.023
Pang XF, Deng B (2008) The changes of macroscopic features and microscopic structures of water under influence of magnetic field. Physica B 403:3571–3577. https://doi.org/10.1016/j.physb.2008.05.032
Qu Z, Li MJ, Wang QJ, Sun Y, Su LJ, Li J (2020) Effect of micro-nano oxygenated water addition on nitrification of Xinjiang sandy loam soil under controlled conditions. Trans Chin Soc Agric Eng 36:189–196. https://doi.org/10.11975/j.issn.1002-6819.2020.22.021 (In Chinese)
Radhakrishnan R, Ranjitha KBD (2012) Pulsed magnetic field: a contemporary approach offers to enhance plant growth and yield of soybean. Plant Physiol Bioch 51:139–144. https://doi.org/10.1016/j.plaphy.2011.10.017
Shao T, Zhao JJ, Liu A, Long X, Rengel Z (2020) Effects of soil physicochemical properties on microbial communities in different ecological niches in coastal area. Appl Soil Ecol 150:103486. https://doi.org/10.1016/j.apsoil.2019.103486
Shen W, Ni Y, Gao N, Bian B, Zheng S, Lin X, Chu H (2016) Bacterial community composition is shaped by soil secondary salinization and acidification brought on by high nitrogen fertilization rates. Appl Soil Ecol 108:76–83. https://doi.org/10.1016/j.apsoil.2016.08.005
Suyal DC, Soni R, Goel R (2021) Microbiome change of agricultural soil under organic farming practices. Biologia 76(4):1315–1325. https://doi.org/10.2478/s11756-021-00680-6
Tian W, Wang L, Li Y, Zhuang K, Li G, Zhang J, Xiao X, Xi Y (2015) Responses of microbial activity, abundance, and community in wheat soil after three years of heavy fertilization with manurebased compost and inorganic nitrogen. Agr Ecosyst Environ 213:219–227. https://doi.org/10.1016/j.agee.2015.08.009
Tripathi BM, Stegen JC, Kim M, Dong K, Adams JM, Lee YK (2018) Soil pH mediates the balance between stochastic and deterministic assembly of bacteria. ISME J 12:1072. https://doi.org/10.1038/s41396-018-0082-4
Wang QJ, Sun Y, Ning SR, Zhang JH, Zhou BB, Su LJ, Shan YY (2019) Effects of activated irrigation water on soil physicochemical properties and crop growth and analysis of the probable pathway. Adv Earth Sci 34(6):660–670. (in Chinese) CNKI:SUN:DXJZ.0.2019–06–014
Wang SY et al (2020) Anaerobic ammonium oxidation is a major N-sink in aquifer systems around the world. ISME J 14(1):151–163. https://doi.org/10.1038/s41396-019-0513-x
Ward NL, Challacombe JF, Janssen PH, Henrissat B, Kuske CR (2009) Three genomes from the phylum acidobacteria provide insight into the lifestyles of these microorganisms in soils. Appl Environ Microb 75:2046–2056. https://doi.org/10.1128/AEM.02294-08
Wardle DA, Bardgett RD, Klironomos JN, Setälä H, Van Der Putten WH, Wall DH (2004) Ecological linkages between aboveground and belowground biota. Science 304:1629–1633. https://doi.org/10.1126/science.1094875
Wei K, Zhang JH, Wang QJ, Chen Y, Ding Q (2021) Effects of ionized brackish water and polyacrylamide application on infiltration characteristics and improving water retention and reducing soil salinity. Can J Soil Sci 101(2):324–334. https://doi.org/10.1139/CJSS-2020-0099
Wei K, Zhang JH, Wang QJ, Guo Y, Mu WY (2022) Irrigation with ionized brackish water affects cotton yield and water use efficiency. Ind Crop Prod 175:114244. https://doi.org/10.1016/j.indcrop.2021.114244
Xiao W, Chen X, Jing X, Zhu B (2018) A meta-analysis of soil extracellular enzyme activities in response to global change. Soil Biol Biochem 123:21–32. https://doi.org/10.1016/j.soilbio.2018.05.001
Xiao EZ, Ning ZP, Xiao TF, Sun WM, Jiang SM (2021) Soil bacterial community functions and distribution after mining disturbance. Soil Biol Biochem 157:108232. https://doi.org/10.1016/j.soilbio.2021.108232
Yafuso EJ, Fisher PR (2018) Oxygenation of irrigation water during propagation and container production of bedding plants. Hortscience 52(11):1608–1614. https://doi.org/10.21273/HORTSCI12181-17
Yang Y, Cheng H, Gao H, An S (2020) Response and driving factors of soil microbial diversity related to global nitrogen addition. Land Degrad Dev 31:190–204. https://doi.org/10.1002/ldr.3439
Ye D, Jiang YH, Yang Y, He Z, Luo F, Zhou J (2012) Molecular ecological network analyses. BMC Bioinformatics 13:113. https://doi.org/10.1186/1471-2105-13-113
Yuan JH, Xu RK, Qian W, Wang RH (2011) Comparison of the amelioration effects on an acidic ultisol between four crop straws and their biochars. J Soil Sediment 11:741–750. https://doi.org/10.1007/s11368-011-0365-0
Zhang H, Sekiguchi Y, Hanada S, Hugenholtz P, Kim H, Kamagata Y, Nakamura K (2003) Gemmatimonas aurantiaca gen. nov., sp. nov., a gram-negative, aerobic, polyphosphate-accumulating micro-organism, the first cultured representative of the new bacterial phylum Gemmatimonadetes phyl. nov. Int J Syst Evol Microbiol 53:1155–1163. https://doi.org/10.1099/ijs.0.02520-0
Zhang JH, Wang QJ, Wei K, Guo Y, Mu WY, Sun Y (2022) Magnetic water treatment: an eco-friendly irrigation alternative to alleviate salt stress of brackish water in seed germination and early seedling growth of cotton (Gossypium hirsutum L). Plants-Basel 11(11):1397. https://doi.org/10.3390/plants11111397
Zhao FY, Sun JL, Jiang Y, Hu DJ, Yang X, Dong MM, Yu K, Yu SL (2018a) Effect of rhizosphere aeration by subsurface drip irrigation with tanks on the growth of “Red Globe” grape seedling and its absorption, distribution and utilization of urea-N15. Sci Hortic 236:207–213. https://doi.org/10.1016/j.scienta.2018.03.041
Zhao S, Liu JJ, Banerjee S, Zhou N, Zhao ZY, Zhang K, Tian CY (2018b) Soil pH is equally important as salinity in shaping bacterial communities in saline soils under halophytic vegetation. Sci Rep-UK 8:4550. https://doi.org/10.1038/s41598-018-22788-7
Zhao GQ, Zhou BB, Mu Y, Wang YH, Liu YQ, Wang L (2022) Irrigation with activated water promotes root growth and improves water use of winter wheat. Agronomy-Basel 11(12):2459. https://doi.org/10.3390/agronomy11122459
Zheng W, Xue DM, Li XZ, Ye D, Wang ZL (2017) The responses and adaptations of microbial communities to salinity in farmland soils: a molecular ecological network analysis. Appl Soil Ecol 120:239–246. https://doi.org/10.1016/j.apsoil.2017.08.019
Zhou Z, Wang C, Zheng M, Jiang L, Luo Y (2017) Patterns and mechanisms of responses by soil microbial communities to nitrogen addition. Soil Biol Biochem 115:433–441. https://doi.org/10.1016/j.soilbio.2017.09.015
Zhou BB, Yang L, Chen XP, Ye ST, Peng Y, Liang CF (2021a) Effect of magnetic water irrigation on the improvement of salinized soil and cotton growth in Xinjiang. Agr Water Manage 248:106784. https://doi.org/10.1016/j.agwat.2021.106784
Zhou LZ, Zhou FX, Ying S, Li SY (2021b) Study on water and salt migration and deformation properties of unsaturated saline soil under a temperature gradient considering salt adsorption: numerical simulation and experimental verification. Comput Geotech 134:104094. https://doi.org/10.1016/j.compgeo.2021.104094
Zhou BB, Liang CF, Chen XP, Ye ST, Peng Y, Yang L, Duan ML, Wang XP (2022) Magnetically-treated brackish water affects soil water-salt distribution and the growth of cotton with film mulch drip irrigation in Xinjiang, China. Agr Water Manage 263:107487. https://doi.org/10.1016/j.agwat.2022.107487
Zhu MJ, Wang QJ, Sun Y, Zhang JH (2021) Effects of oxygenated brackish water on germination and growth characteristics of wheat. Agr Water Manage 245:106520. https://doi.org/10.1016/j.agwat.2020.106520
Funding
This work was financed by the National Natural Science Foundation of China (41907010, 41830754, 41977043), Key Science-Technology Project of Inner Mongolia (2021GG0251), and Special Project of Natural Science of Shaanxi Provincial Department of Education (21JK0783).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, Y., Wang, C., Mi, W. et al. Effects of Irrigation Using Activated Brackish Water on the Bacterial Community Structure of Rhizosphere Soil. J Soil Sci Plant Nutr 22, 4008–4023 (2022). https://doi.org/10.1007/s42729-022-01003-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-022-01003-7