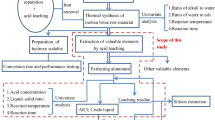
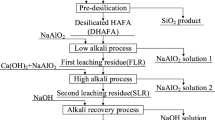
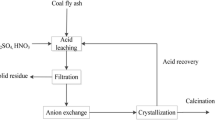
Abstract
The rapid development of the global alumina and aluminum industry is straight coupled with the demands and the needs of these materials. Nowadays, the only well-known accessible resources to produce alumina and aluminum are bauxite ores. But bauxite ores are limited, and the remaining exploitable reserves do not have high contents in alumina. In countries where there is little or no bauxite reserves or where the recovery of existing bauxite ores to produce alumina with the Bayer process are not economic, alternative resources for alumina have been explored. The most attractive resources for replacing bauxites are aluminosilicate clay minerals and coal fly ash. The important processes developed for the recovery of alumina from these sources are acid leaching and alkaline leaching processes. The acid leaching is known as the most efficient in which thermal and mechanical activation are included for improving the performance of the process. The objective of this review is to give a comprehensive insight on the production of the alumina from thermally or mechanically activated clays and coal fly ash by hydrochloric, sulfuric, and nitric acid leaching processes.










Similar content being viewed by others
References
Bray EL (2011) Bauxite and alumina. Min Eng 63(6):44–45
Brubaker S (1967) Trends in the world aluminum industry. Nat Resour J 8(4):744–746
World Aluminium (2018) Primary aluminium production. www.world-aluminium.org/statistics/. Accessed 15 August 2018
Bazin C, El-Ouassiti K, Ouellet V (2007) Sequential leaching for the recovery of alumina from a Canadian clay. Hydrometallurgy 88(1-4):196–201
Erdemoğlu M, Birinci M, Uysal T (2018) Alumina production from clay minerals: current reviews. J Polytech 21(2):387–396
Qiu G, Jiang T, Li G, Fan X, Huang Z (2004) Activation and removal of silicon in kaolinite by thermochemical process. Scand J Metall 33(2):121–128
Al-Sindy SI, Al-Ajeel AWA (2006) Alumina recovery from Iraqi kaolinitic clay by hydrochloric acid route. Iraq Bull Geol Min 2(1):67–76
Kinnarinen T, Holliday L, Häkkinen A (2015) Dissolution of sodium, aluminum and caustic compounds from bauxite residues. Miner Eng 79:143–151
Erdemoğlu M, Birinci M, Uysal T, Tüzer EP, Barry TS (2018) Mechanical activation of pyrophyllite ore for aluminum extraction by acidic leaching. J Mater Sci 53(19):13801–13,812
Hixson AW, Ralph M (1945) Production of alumina United States Patents No: 2376696A
Lowenstein HM, Lowenstein AM (1976) Alumina production United States Patents No: 3983212A
Lisowyj B (1986) Method for extraction of iron aluminum and titanium from coal ash United States Patents No: 4567026A
Gaudernack B, Gjelsvik N, Farbu L (1978) Process for the extraction of alumina from aluminum-containing silicates United States Patents No: 4110399A
Krauß D (1978) Dossiers III.: Aluminium. Raw Materials Research and Development, November 1978
Baudet G (1977) A documentary study on alumina extraction processes. https://vdocuments.mx/a-documentary-study-on-alumina-extraction-processes-documentary-study-on-alumina.html. Accessed 15 August 2018
Ding J, Ma S, Shen S, Xie Z, Zheng S, Zhang Y (2017) Research and industrialization progress of recovering alumina from fly ash: a concise review. Waste Manag 60:375–387
Habashi F (2017) Hydrochloric acid in hydrometallurgy. Proceeding of the 56th Annual Conference of Metallurgists, Hosting World Gold and Nickel-Cobalt in Hyatt Regency Vancouver – British Columbia, Canada. August 2017
Liu K, Xue J, Zhu J (2012) Extracting alumina from coal fly ash using acid Sintering-leaching process. In: Suarez CE (ed) Light metals 2012. Springer, Cham, pp 201–206
Yao Z, Xia M, Sarker PK, Chen T (2014) A review of the alumina recovery from coal fly ash, with a focus in China. Fuel 120:74–85
Zhu P-w, Dai H, Han L, Xu X-l, Cheng L-m, Wang Q-h, Z-l S (2015) Aluminum extraction from coal ash by a two-step acid leaching method. J Zhejiang Univ Sci A 16(2):161–169
Habashi F (2017) Alumina from silicates. Proceeding of 35th ISCOBA Conference and Exhibition in Hamburg. Newsletter Volume 17, June 2017
Hosterman JW, Patterson SH, Good EE (1990) World nonbauxite aluminium resources excluding alunite
España VAA, Sarkar B, Biswas B, Rusmin R, Naidu R (2016) Environmental applications of thermally modified and acid activated clay minerals: current status of the art. Environ Technol Innov https://doi.org/10.1016/j.eti.2016.11.005
Sadik C, El Amrani I-E, Albizane A (2014) Recent advances in silica-alumina refractory: a review. J Asian Ceramic Soc 2(2):83–96
Panda AK, Mishra BG, Mishra DK, Singh RK (2010) Effect of sulphuric acid treatment on the physico-chemical characteristics of kaolin clay. Colloids Surf A Physicochem Eng Asp 363(1-3):98–104
Li H, Hui J, Wang C, Bao W, Sun Z (2014) Extraction of alumina from coal fly ash by mixed-alkaline hydrothermal method. Hydrometallurgy 147:183–187
Shemi A, Mpana R, Ndlovu S, Van Dyk L, Sibanda V, Seepe L (2012) Alternative techniques for extracting alumina from coal fly ash. Miner Eng 34:30–37
Robie RA, Hemingway BS (1995) Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar (105 Pascals) pressure and at higher temperatures. US Government Printing Office
Gaboreau S, Vieillard P (2004) Prediction of Gibbs free energies of formation of minerals of the alunite supergroup. Geochim Cosmochim Acta 68(16):3307–3316
Numluk P, Chaisena A (2012) Sulfuric acid and ammonium sulfate leaching of alumina from Lampang clay. J Chem 9(3):1364–1372
Hosseini SA, Niaei A, Salari D (2011) Production of γ-Al2O3 from Kaolin. Open J Phys Chem 1(02):23–27
San Cristóbal AG, Castelló R, Luengo MAM, Vizcayno C (2009) Acid activation of mechanically and thermally modified kaolins. Mater Res Bull 44(11):2103–2111
Tang A, Su L, Li C, Wei W (2010) Effect of mechanical activation on acid-leaching of kaolin residue. Appl Clay Sci 48(3):296–299
de Carvalho Costa TC, Melo JDD, Paskocimas CA (2013) Thermal and chemical treatments of montmorillonite clay. Ceram Int 39(5):5063–5067
Cheng F, Cui L, Miller J, Wang X (2012) Aluminum leaching from calcined coal waste using hydrochloric acid solution. Miner Process Extr Metall Rev 33(6):391–403
Guo Y, Yan K, Cui L, Cheng F (2016) Improved extraction of alumina from coal gangue by surface mechanically grinding modification. Powder Technol 302:33–41
Xiao J, Li F, Zhong Q, Bao H, Wang B, Huang J, Zhang Y (2015) Separation of aluminum and silica from coal gangue by elevated temperature acid leaching for the preparation of alumina and SiC. Hydrometallurgy 155:118–124
D'Elia A, Pinto D, Eramo G, Giannossa L, Ventruti G, Laviano R (2018) Effects of processing on the mineralogy and solubility of carbonate-rich clays for alkaline activation purpose: mechanical, thermal activation in red/ox atmosphere and their combination. Appl Clay Sci 152:9–21
Li G, Zeng J, Luo J, Liu M, Jiang T, Qiu G (2014) Thermal transformation of pyrophyllite and alkali dissolution behavior of silicon. Appl Clay Sci 99:282–288
Birinci M, Uysal T, Erdemoğlu M, Porgalı E, Barry T (2017) Acidic leaching of thermally activated pyrophyllite ore from Puturge (Malatya-Turkey) deposit. Proceeding of XVII Balkan Mineral Processing Congress, Antalya
Habashi F (1999) A textbook of hydrometallurgy. Métallurgie Extractive
Baláž P (2003) Mechanical activation in hydrometallurgy. Int J Miner Process 72(1):341–354
Erdemoğlu M, Baláž P (2012) An overview of surface analysis techniques for characterization of mechanically activated minerals. Miner Process Extr Metall Rev 33(1):65–88
Melo JDD, de Carvalho Costa TC, de Medeiros AM, Paskocimas CA (2010) Effects of thermal and chemical treatments on physical properties of kaolinite. Ceram Int 36(1):33–38
Fabbri B, Gualtieri S, Leonardi C (2013) Modifications induced by the thermal treatment of kaolin and determination of reactivity of metakaolin. Appl Clay Sci 73:2–10
Luo J, Jiang T, Li G, Peng Z, Rao M, Zhang Y (2017) Porous materials from thermally activated kaolinite: preparation, characterization and application. Materials 10(6):647
Nones J, Nones J, Riella HG, Poli A, Trentin AG, Kuhnen NC (2015) Thermal treatment of bentonite reduces aflatoxin b1 adsorption and affects stem cell death. Mater Sci Eng C 55:530–537
Murat M, Driouche M (1988) Chemical reactivity of thermally activated clay minerals estimation by dissolution in hydrofluoric acid. Cem Concr Res 18(2):221–228
Uysal T (2018) Investigation of activation conditions in alumina production from pyrophyllite ore by acid leaching method. PhD Thesis, Inonu University, Mining Engineering Department, Malatya, Turkey
Tkáčová K, Baláž P, Mišura B, Vigdergauz V, Chanturiya V (1993) Selective leaching of zinc from mechanically activated complex Cu-Pb-Zn concentrate. Hydrometallurgy 33(3):291–300
Tkáčová K, Baláž P (1996) Reactivity of mechanically activated chalcopyrite. In: Comminution 1994. Elsevier, pp 197-208
Baláž P, Boldižárová E, Achimovičová M, Kammel R (2000) Leaching and dissolution of a pentlandite concentrate pretreated by mechanical activation. Hydrometallurgy 57(1):85–96
Achimovičová M, Baláž P (2005) Influence of mechanical activation on selectivity of acid leaching of arsenopyrite. Hydrometallurgy 77(1-2):3–7
Zhang Y, Li X, Pan L, Wei Y, Liang X (2010) Effect of mechanical activation on the kinetics of extracting indium from indium-bearing zinc ferrite. Hydrometallurgy 102(1):95–100
X-h L, Y-j Z, Pan L-p, Y-s W (2013) Effect of mechanical activation on dissolution kinetics of neutral leach residue of zinc calcine in sulphuric acid. Trans Nonferrous Metals Soc China 23(5):1512–1519
Baláž P, Dutková E (2009) Fine milling in applied mechanochemistry. Miner Eng 22(7-8):681–694
Baláž P, Aláčová A, Achimovičová M, Ficeriova J, Godočíková E (2005) Mechanochemistry in hydrometallurgy of sulphide minerals. Hydrometallurgy 77(1-2):9–17
Erdemoğlu M, Aydoğan S, Gock E (2009) Effects of intensive grinding on the dissolution of celestite in acidic chloride medium. Miner Eng 22(1):14–24
Erdemoğlu M (2009) Carbothermic reduction of mechanically activated celestite. Int J Miner Process 92(3-4):144–152
Kalinkin AM, Kalinkina EV, Makarov VN (2003) Mechanical activation of natural titanite and its influence on the mineral decomposition. Int J Miner Process 69(1-4):143–155
Wang X, Li C, Yue H, Yuan S, Liu C, Tang S, Liang B (2018) Effects of mechanical activation on the digestion of ilmenite in dilute H2SO4. Chin J Chem Eng. https://doi.org/10.1016/j.cjche.2018.06.020
Kumar S, Kumar R (2011) Mechanical activation of fly ash: effect on reaction, structure and properties of resulting geopolymer. Ceram Int 37(2):533–541
Kumar S, Mucsi G, Kristaly F, Pekker P (2017) Mechanical activation of fly ash and its influence on micro and nano-structural behaviour of resulting geopolymers. Adv Powder Technol 28(3):805–813
Zhang J, Yan J, Sheng J (2015) Dry grinding effect on pyrophyllite–quartz natural mixture and its influence on the structural alternation of pyrophyllite. Micron 71:1–6
Ohale PE, Uzoh CF, Onukwuli OD (2017) Optimal factor evaluation for the dissolution of alumina from Azaraegbelu clay in acid solution using RSM and ANN comparative analysis. S Afr J Chem Eng 24:43–54
Bai G, Qiao Y, Shen B, Chen S (2011) Thermal decomposition of coal fly ash by concentrated sulfuric acid and alumina extraction process based on it. Fuel Process Technol 92(6):1213–1219
Shemi A, Ndlovu S, Sibanda V, van Dyk L (2015) Extraction of alumina from coal fly ash using an acid leach-sinter-acid leach technique. Hydrometallurgy 157:348–355
Sokolova TA (2013) Decomposition of clay minerals in model experiments and in soils: possible mechanisms, rates, and diagnostics (analysis of literature). Eurasian Soil Sci 46(2):182–197
Kittrick JA (1969) Soil Minerals in the Al2O3-SiO2-H2O system and a theory of their formation. Clay Clay Miner 17:157–167
Schott J, Oelkers EH (1995) Dissolution and crystallization rates of silicate minerals as a function of chemical affinity. Pure Appl Chem 67(6):903–910
Ganor J, Mogollon JL, Lasaga AC (1995) The effect of pH on kaolinite dissolution rates and on activation energy. Geochim Cosmochim Acta 59(6):1037–1052
Huertas FJ, Chou L, Wollast R (1998) Mechanism of kaolinite dissolution at room temperature and pressure: Part I. Surface speciation. Geochim Cosmochim Acta 62(3):417–431
Huertas FJ, Chou L, Wollast R (1999) Mechanism of kaolinite dissolution at room temperature and pressure Part II: Kinetic study. Geochim Cosmochim Acta 63(19/20):3261–3275
Cama J, Metz V, Ganor J (2002) The effect of pH and temperature on kaolinite dissolution rate under acidic conditions. Geochim Cosmochim Acta 66(22):3913–3926
Saldi GD, Köhler SJ, Marty N, Oelkers EH (2007) Dissolution rates of talc as a function of solution composition, pH and temperature. Geochim Cosmochim Acta 71:3446–3457
Krauskopf KB, Bird DK (1995) Introduction to Geochemistry, 3rd edn. McGraw-Hill, Inc., NewYork
Daniels AL, Muzenda E (2012) Recovery of aluminium oxide from flint clay through H2SO4 leaching. Proceedings of the World Congress on Engineering Vol III WCE 2012, July 4 - 6, 2012, London, U.K.
Dewey JL, Scott CE, Kane JF, Stratton CL, Rushing JC, Spoonts RH (1981) Alumina production by nitric acid extraction of clay United States Patents No: 4246239A
Redlich O, March CC, Adams MF, Sharp FH, Holt EK, Taylor JE (1946) Extraction of alumina from clay. Ind Eng Chem 38(11):1181–1187
Valeev D, Lainer YA, Samokhin A, Sinayskiy M, Mikhailova A, Kutsev S, Goldberg M (2016) Physicochemical studies on the thermal hydrolysis of aluminum chloride. Inorg Mater Appl Res 7(5):779–785
Si P, Qiao X, Yu J (2012) Alumina recovery from kaolin with mineral impurities. J Wuhan Univ Technol 27(6):1139–1143
Tomaino G (2000) Talc and pyrophyllite. Min Eng 52(6):64–65
Çılgı G, Cetişli H (2009) Thermal decomposition kinetics of aluminum sulfate hydrate. J Therm Anal Calorim 98(3):855–861
Yarkadas G, Yildiz K (2008) Effects of mechanical activation on alumina extraction from alunite ore and its thermal behaviour. Miner Process Ext Metall 117(3):175–178
Seidel A, Zimmels Y (1998) Mechanism and kinetics of aluminum and iron leaching from coal fly ash by sulfuric acid. Chem Eng Sci 53(22):3835–3852
El-Shereafy E, Abousekkina M, Mashaly A, El-Ashry M (1998) Mechanism of thermal decomposition and γ-pyrolysis of aluminum nitrate nonahydrate [Al(NO3)3·9H2O]. J Radioanal Nucl Chem 237(1-2):183–186
Peters FA, Kirby R, Higbie K (1967) Methods for producing alumina from clay—an evaluation. JOM 19(10):26–34
Hulbert S, Huff D (1970) Kinetics of alumina removal from a calcined kaolin with nitric, sulphuric and hydrochloric acids. Clay Miner 8(3):337–345
Acknowledgements
The authors would appreciatively like to acknowledge the Scientific and Technological Research Council of Turkey (TÜBİTAK) for financing this study by the project with grant No. 214M432 and İnönü University Scientific Research Projects Unit for support with grant No 2015/44G.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Barry, T.S., Uysal, T., Birinci, M. et al. Thermal and Mechanical Activation in Acid Leaching Processes of Non-bauxite Ores Available for Alumina Production—A Review. Mining, Metallurgy & Exploration 36, 557–569 (2019). https://doi.org/10.1007/s42461-018-0025-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-018-0025-7