Abstract
Strategies involving the synthesis of hydroxyapatite (HAP) are significant in health and materials sciences, and lately, in heritage science. Conditions for the optimized heterogeneous formation of HAP for consolidating bone are investigated using a simple methodology with discs of calcium-containing starting materials and phosphate-containing reagent solutions; the results were evaluated against whole bone specimens. Diammonium phosphate (DAP) solutions with simulated body fluid (SBF) and gelatin as cofactors in all combinations were used in trials, while the products were characterized by microscopy, elemental microanalysis, X-Ray diffractometry, and Fourier transform infrared spectroscopy. The hypothesis that bone, SBF, and gelatin may biomimetically promote HAP formation was tested through a simple experimental design. The results showed that HAP is generally formed in mixtures with octacalcium phosphate (OCP), as well as calcium hydrogen phosphate dihydrate (brushite). The precipitation of carbonate HAP signals the formation of a biocompatible product in the cases where SBF and gelatin were employed as cofactors. Gelatin was found to further promote the product formation in most cases. It was shown that DAP, as well as its combination with gelatin, could predominantly form HAP in most trials, while OCP is co-crystallized in SBF-containing solutions.
Similar content being viewed by others
1 Introduction
In the protection of cultural heritage, methodologies have been developed employing chemical strategies for synthesizing minerals such as organo-silicates, oxalates, and hydroxyapatite (HAP) for the consolidation of limestone and marble-containing objects in cultural heritage [1,2,3,4,5,6]; bone has been recently added to the list [7].
Bone is a composite material consisting of an organic fraction (mainly collagen and smaller amounts of non-collagenous proteins, lipids, and proteoglycans), an inorganic matrix (carbonate hydroxyapatite, or c-HAP), and water (structural, bound, free, and adsorbed) [8,9,10,11]. Structurally, it consists of small, elongated plate-like mineral crystals (up to 100 nm long, 2–4 nm thick) embedded in the protein matrix [9, 12, 13].
Archaeological bone in its burial environment can seriously deteriorate through diagenesis, a complex process [9, 14,15,16,17] that leads to the damaging (a) of collagen by the action of micro-organisms and hydrolytic degradation and (b) of the inorganic matrix due to slow solubilization and recrystallization of minerals. The deterioration of any of the above two fractions exposes the other, thus affecting their profiles and crystallinity [9, 17,18,19,20,21]. Conventional approaches for bone consolidation involve the use of natural (wax, resins, gums) and synthetic materials (acrylic resins, polyvinyl acetals, and butyrals). These approaches, however, suffer from poor or uncontrolled penetration into the bone matrix, and therefore, offer temporary and sometimes problematic protection, potentially leading to severe damage due to yellowing, embrittlement, shrinkage, and delamination with time [7, 22].
The in situ formation of HAP on the surface of bone has been proposed as a biomimetic consolidation strategy [7, 23], using water-soluble diammonium phosphate (DAP) as a phosphate source on the one hand, and the bone surface as a calcium source and bio-scaffold simultaneously, on the other, for the synthesis and deposition of highly insoluble HAP. According to this strategy, in situ-formed HAP microcrystals can bridge ruptured or discontinued bone matrix phases, thus offering consolidation of the material.
A vital component of bone in vertebrates, hydroxyapatite, is one of the most common minerals in the living world [9, 24, 25]. Its synthetic formation has been widely exploited towards technological solutions in health and related materials sciences [26,27,28,29,30]. Stoichiometric HAP (s-HAP) can be formed according to the following reaction scheme, involving a calcium salt as a source of Ca2 + and hydrogen phosphate (HPO42−):
During the reaction, HAP, as the most thermodynamically stable form, precipitates according to a path that involves tricalcium phosphate (Ca3(PO4)2), brushite (CaHPO4⋅ 2H2O) and octacalcium phosphate (Ca8H2(PO4)6 ⋅ 5H2O) as precursor phases [6, 31,32,33] (see Table 1). During HAP synthesis, the characterization of the actual phosphate product depending on the conditions (the type of ionic solution, pH, time of application), in combination with a conclusive analytical investigation, is crucial.
For evaluating the successful formation of stoichiometric HAP, Scanning Electron Microscopy with Energy Dispersive X-Ray Analysis (SEM–EDX), X-ray diffractometry (XRD), and Fourier Transform Infrared Spectroscopy (FTIR) is typically employed. A first criterion is the Ca/P ratio, which is typically estimated through microelemental elemental analysis (SEM–EDX), which, for s-HAP, is 1.67. Deviation from this value may suggest that other phosphate phases are also produced, such as nanocrystalline and carbonate hydroxyapatite (n-HAP and c-HAP, respectively) as well as octacalcium phosphate (OCP).
X-ray diffractometry (XRD) and Fourier Transform Infrared Spectroscopy (FTIR) are the main techniques for characterizing the condition of archaeological bone in the burial environment [34,35,36,37,38,39], as well as HAP, in particular [13, 30]. In s-HAP, peaks are well-resolved, while in n-HAP and c-HAP, imperfections in the crystal lattice are reflected in broader envelopes [31, 40]. In XRD spectra, the more prominent peaks of (211), (112), (300) and (202) crystalline planes with d-spacings at 3.32, 2.93, 2.79 and 2.72 Å, respectively, are broadened and sometimes overlapping [31] (see, for example, spectra recorded through the trials of this work in Fig. 2c–d). Additionally, line shape analysis of XRD peaks provides information on crystallinity; a widely accepted methodology involves the correlation of the Full-Width Half Maximum (FWHM) of spectra with crystal size [41,42,43].
Similarly, in FTIR spectra, the two components of the v3 phosphate vibrational mode are mutually overlapping, allowing a maximum at ~ 1020 cm−1 and a shoulder at 1100 cm−1 (see spectra ii and v in Fig. 2a) [44, 45]. On the other hand, the v4 vibrational mode is typically observed as a resolved doublet, in which, the ‘valley’ between the doublet components (604 and 563 cm−1, as seen in the same spectra), typically expressed as the infrared splitting factor (IRSF), is often used to assess the crystallinity of HAP [1, 13, 25, 34, 46,47,48,49]. In bone c-HAP, carbonates occur in two distinct types: type A for those replacing hydroxide ions of the stoichiometric HAP structure (with a maximum of their v3 vibration at ~ 1456 cm−1), and type B for those replacing phosphates (at ~ 1415 cm−1) [31, 40, 50,51,52,53] as seen in Fig. 1. Values and assignments are listed in Table 2. Semi-quantification of carbonate in c-HAP can be achieved by calculating the carbonate index, i.e. the ratio of the type B carbonate absorption height over that of phosphate v3 [49, 54, 55]. Also, an estimate of the type A/type B ratio can be obtained by comparing the areas under the deconvoluted components of the v2 carbonate vibration at 880 cm−1 (type A) and 873 (type B) [21, 56].
Laboratory synthesis of HAP has been achieved following various methods such as wet chemical precipitation from calcium hydroxide and phosphoric acid as well as multiphase systems in hydrothermal and sol–gel synthesis methods [1, 43, 57,58,59,60,61]. Additionally, biomimetic approaches have been developed, aiming at forming various apatites, mainly for health applications, such as bone regeneration [27, 62, 63] and bio-compatible coatings for bone and dental implants [64,65,66]. In this aspect, wet chemical routes based on Simulated body fluid (SBF), a supersaturated cocktail that resembles the ionic content of blood serum, has been proposed [57, 59, 62, 67]. The SBF approach is adopted as an ionic environment that promotes the synthesis of HAP [58, 59, 62, 67, 68], while protocols for its preparation are generally under scrutiny and being constantly revised [27, 31, 62, 69,70,71].
Alternatively, strategies involving molecular scaffolds that facilitate the right ionic profile towards HAP, thus producing HAPs structurally and morphologically similar to those in bone, have also been proposed [2, 7, 64].
This work aims at investigating the working conditions for efficient in situ precipitation of hydroxyapatite on the surface of bone aiming at its consolidation. A simple experimental methodology is applied, employing the biomimetic scaffold offered by bone, in combination with cofactors such as SBF and gelatin. The compatibility concept is also questioned as to whether nanocrystalline carbonate hydroxyapatite, similar to the one existing in bone, can be formed.
2 Materials and techniques
2.1 Materials
Water, HPLC grade, was purchased from Honeywell. CaCO3, CaCl2, and NaCl were purchased from Merck. Diammonium phosphate (DAP) Disodium phosphate (Na2HPO4) was purchased from Sigma-Aldrich. HCl (36% w/v) was purchased from Fluka.
2.2 Trials for the formation of hydroxyapatite
2.2.1 Preparation of specimens
Hydraulic press For the preparation of discs, a standard 13 mm evacuable pellet die and disc holder with rectangular mount, and a 15 ton (max) manual hydraulic press, all provided by Specac (Orpington, Kent, UK) were used.
13-mm diameter disc specimens: (a) Calcium carbonate(calcite, Merck) powder was manually finely ground with a pestle and mortar and (b) bone powder was prepared by gently grinding young Roe deer bone (kindly offered by DRAFA, Denmark) with a motorized grinder using the lowest speed available to avoid the release of excessive heat.
These were accordingly pressed and formed to 13-mm discs using a standard FTIR-disc mold and after hydraulic pressing at 10 and 3 tn, respectively.
Whole bone specimens: a seagull leg bone (found in presumably naturally aged condition in the open air, in which had remained for an unspecified period) was cut in approximately 15 mm-long pieces of variable thickness and were consequently used without further workup.
2.2.2 SBF-based HAP synthesis
2.2.2.1 Preparation of simulated body fluid (SBF)
SBF was prepared in HPLC-grade water by sequentially mixing NaCl (0.3319 g), Na2HPO4 (0.0071 g), NaHCO3 (0.1134 g), Na2SO4 (0.0035 g), KCl (0.1862 g), CaCl2 (0.0138 g) and MgCl2.6H2O (0.0152 g), with until 50 ml final volume with no further stirring. The pH was regulated at 7.3–7.5 by adding hydrochloric acid (1 M, dropwise until pH 7.3–7.5). The above route was based on previous work [31, 58, 59, 69]. This way, a solution containing ions in the required concentrations was prepared and used as required (see Sect. 3.1).
2.2.2.2 Synthesis of HAP using SBF
The precipitation of HAP was generally achieved by adding a calcium salt (CaCl2) and a phosphate source in the form of water-soluble salt: Na2HPO4 (disodium phosphate or DSP and (NH4)2HPO4 (diammonium phosphate or DAP) to previously prepared SBF solutions; the reaction proceeds according to Eq. 1.
Several variations were considered and attempted, from which, selected trials are herewith presented:
2.2.2.3 DSP as a phosphate source
Stock solutions of CaCl2 (0.5 M, by adding 1.3875 g of solid CaCl2 in 10 ml of H2O) and DSP (0.3 M, by adding 1.3349 g of solid Na2HPO4 in 10 ml H2O) were prepared.
Trial SBF-1: To 25 ml of SBF solution (see above), 5 ml of the 0.5 M CaCl2 solution was added, followed by dropwise addition (1 drop/10 s, approx.) of the 0.3 M DSP solution at 36.5° C with no stirring, until a cloudy suspension is formed.
Trial SBF-2: To 25 ml of SBF solution, 10 ml of the 0.5 M CaCl2 solution was added, followed by dropwise addition (1 drop/10 s, approx.) of the 0.3 M DSP solution at 36.5° C under continuous stirring until 10 ml of the phosphate was consumed.
2.2.2.4 DAP as a phosphate source
Stock solutions of CaCl2 (1.25 M, by adding 1.3875 g of solid CaCl2 in 10 ml of H2O) and (NH4)2HPO4 or diammonium phosphate, DAP (0.75 M by adding 0.99 g of solid (NH4)2HPO4 in 10 ml of H2O) were prepared.
Trial SBF-3: To 25 ml of SBF solution, 10 ml of the 1.25 M CaCl2 solution was added followed by dropwise addition (1 drop/5 s, approx.) of the 0.75 M DAP solution at 36.5° C under continuous stirring until 10 ml of the phosphate was consumed (attempt No8).
Trial SBF-3: To 25 ml of SBF solution, 10 ml of the 1.25 M CaCl2 solution was added followed by dropwise addition (1 drop/5 s, approx.) of the 0.75 M DAP solution at 36.5 °C under continuous stirring until 10 ml of the phosphate was consumed (attempt No8).
SBF-5: To 25 ml of SBF solution, 0.1 g of gelatin was added. Then, 5 ml of the 1.25 M CaCl2 solution was added, followed by dropwise addition (1 drop/5 s, approx.) of the 0.75 M DAP solution at 36.5 °C with no stirring, until a cloudy suspension is formed, and 10 ml of the phosphate was consumed.
Products were characterized by SEM–EDX, XRD, and KBr-FTIR.
2.3 In situ synthesis of hydroxyapatite (HAP) on calcite and bone powder discs
In all trials for in situ deposition of HAP, the following reagent solutions were made: (a) diammonium phosphate (DAP), 0.5 M, solution (prepared by adding 0.33 g of solid (NH4)2HPO4 to 5 ml of H2O); (b) DAP/SBF: to 5 ml of the DAP solution, 5 ml of previously prepared SBF solution (see above) was added; (c) DAP/gelatin: to 5 ml of the DAP solution, 5 ml of 0.1% w/v aqueous gelatin solution was added; (d) DAP/SBF/gelatin: to 5 ml of the DAP solution, 5 ml of previously prepared SBF solution (see above) and 5 ml of 0.1% w/v aqueous gelatin solution was added, 5 ml of 0.1% w/v aqueous gelatin solution was added.
In all cases, 12 mm discs were prepared according to the standard procedure with the use of a dedicated disc mold and a SpecAc (Orpington, Kent, UK) manual hydraulic press.
-
a.
Control trials on calcite discs
In the control experiments, four pressed CaCO3 (calcite) powder discs were prepared (see above). The disc was divided into four approximately equal pieces.
Trials Ca -1 to 4: each of the above-described discs were immersed in a 10 mL DAP (trial Ca-1), a 10 mL DAP/SBF solution (trial Ca-2), a 10 mL DAP/gelatin solution (trial Ca-3), and finally, a 10 mL DAP/SBF/gelatin solution (trial Ca-4). After 5 min immersion time, the discs were removed and accordingly examined with optical microscopy, and characterized with SEM–EDX, ATR-FTIR, and XRD.
Later, additional quantities of the DAP, DAP/SBF, DAP/gelatin, and DAP/SBF/gelatin solutions were respectively added dropwise (3 drops (0.05 ml each) maximum) and the discs were similarly examined and characterized (trials Ca-5, 6 7 and 8, respectively).
-
b.
Trials on bone powder discs
Four pressed 13 mm bone powder discs were prepared (see above) (trials BP-1 to 4).
Trials BP-1 to 4: the previously described discs were treated and accordingly examined and analyzed similarly to the Ca-1 to 4 trials. Products were characterized by SEM–EDX and ATR-FTIR.
-
c.
Trials on Ca2+-enriched pressed bone powder disks
Trials BPCa-1 to 4: Four pressed 13 mm bone powder discs were prepared similarly to those in trials BP-1 to 4 and were calcium-enriched by adding three drops of a 6.5% v/w CaCl2/H2O solution to each; the calcium salt solution is estimated to be in excess with respect to the dripped DAP quantity. Products were characterized by SEM–EDX, XRD, and ATR-FTIR.
2.4 In situ synthesis of hydroxyapatite on calcium-enriched bone specimens
Trials B-1 to 4: four seagull bone specimens were cut from the same part of the bird’s leg bone in approx. 3.0–4.0 mm length and 0.5–1.0 mm diameter; each of these was immersed in DAP, DAP/SBF, DAP/gelatin, and DAP/SBF/gelatin solutions (concentrations the same as above) and left with no stirring for 24 h. Products were characterized by SEM–EDX and KBr-FTIR.
2.5 analysis techniques
2.5.1 Scanning electron microscopy–Energy-dispersive x-ray analysis (SEM–EDX)
In all cases, a JEOL JSM-6510LV Scanning Electron Microscope equipped with an Oxford X-act XEDSM energy dispersive X-Ray analyzer applied vacuum: 40 Pa. Specimens were inserted in the system’s sample chamber without further workup.
2.5.2 Transmission electron microscopy (TEM)
A Thermo-Fisher Scientific Talos F200i Transmission Electron Microscope operating at 200 kV accelerating voltage. and equipped with a window-less Bruker 6 T|100 energy dispersive X-ray micro analyzer was used. The TEM specimens were prepared by scraping off the material to be examined, subsequent grinding by mortar and pestle in ethanol and drop casting 1 μl on copper TEM grids coated with a thin carbon film.
2.5.3 Fourier transform infrared (FTIR) spectroscopy
All samples were analyzed with a Perkin Elmer Spectrum GX spectrometer, equipped with DTGS detector. Spectra were recorded and edited with the PE Spectrum v.5.3.1 software. Further spectra editing was done using the Thermo GRAMS v.9.0 suite. For both KBr and ATR infrared spectra powder samples were used after manual or mechanical grinding (see Materials). The size of bone grains (see Materials and methods) assured secure measurement of the crystallinity index as warned by various researchers [13, 72, 73].
KBr-FTIR spectra: powder samples were mixed with potassium bromide powder (KBr, Merck), finely ground manually with the use of an agate pestle and mortar, and accordingly pressed into 13 mm discs with a hydraulic press. Disc samples were accordingly inserted in the system sample chamber for analysis.
ATR-FTIR spectra: Fourier Transform Infrared Spectroscopy (FT-IR) measurements were carried out using a Pike MIRacle single reflection horizontal ATR accessory with a zinc selenide crystal (4000–525 cm-1) introduced in the sample chamber of the PE Spectrum GX system using the DTGS detector.
Infrared Indices: Spectra in Figs. 1, 2, 3, 4 and 7 are shown as normalized at the v3 phosphate band (~ 1020 cm−1). Collagen and carbonate indices were calculated by the ratios of the absorbance heights at 1660 and 1415 cm−1, correspondingly, over the v3 of phosphate at 1020 cm−1; baseline was set at 1900 and 1380 cm−1. The crystallinity index was measured by calculated Infrared Splitting Factor (IRSF, see Introduction) derived from the doublet of the v4 vibration of hydroxyapatite, according to a standard procedure [13, 46, 49, 74], by using the equation (A604 + A563)/A590, where A corresponds to absorbance heights at the corresponding maxima of the phosphate v4 band. The baseline was set at 800 and 500 cm−1. For all the above calculations of indices, baseline limits may significantly affect the final values [49, 73], and therefore they must be followed thoroughly throughout all the samples. In the present work, the baseline limits by Dal Sasso et al [49]. were adopted. The reproducibility of the method was tested by calculating the average of 7 reference bone samples; std error for collagen index was ± 0.45%, for carbonate index ± 1.24% and for IRSF, ± 1.07%.
a KBr-FTIR spectra of products after SBF-based synthesis in solution; (i) trial SBF-1; (ii) SBF-2; (iii) SBF-3, (iv) SBF-4 and (v) SBF-5. b XRD spectrum of products after trial SBF-1; c XRD spectrum of products after trial SBF-2; d XRD spectrum of products after trial SBF-5. In all XRD spectra, 2θ angles are relative to CoKa1 wavelength
ATR-FTIR spectra zoomed in the 1750–520 cm−1 region, normalized at 1643 cm−1 a of products after trials BP-1, 2, 3, and 4 on pressed bone powder discs followed by water washup, and b of products after trials BPCa-1, 2, 3, and 4, on Ca-enriched bone powder discs, followed by water washup. In both spectra: (0) bone powder before treatment; after immersion in (i) DAP, (ii) DAP/SBF, (iii) DAP/gelatin and (iv) DAP/SBF/gelatin solution. Insets in both Figures: macro-photographs of discs in their initial state, (0), and after each corresponding trial, (i) to (iv)
Deconvolution: deconvolution and peak fitting were applied on the phosphate v4 infrared band using the peak fitting routine in GRAMS/AI software (Thermo). The routine was applied after baseline correction at the limits of the 680–480 cm−1 region of spectra; a Gauss/Lorentz mixed (1:1) model at medium sensitivity and FWHH 8.0 was applied; R2 was better than 0.9990, while std error was lower than 0.0028 in all cases. In Fig. 7b and c, the 2nd derivative is also shown for confirmation of transients.
2.5.4 Stereo-macro-photography
Stereoscopic macro-photographs of discs were recorded using an Olympus 110AL2X-2 WD38 stereomicroscope, using magnifications at × 0.67- × 4.5.
2.5.5 X-ray diffractometry
XRD spectra were recorded using an InXitu BTX II Benchtop X-ray Diffraction/X-ray Fluorescence system using a Cobalt cathode source (Kα1 1.78897 Å). Spectra were analyzed with the InXitu XPowder software and searched using the PDF2 and the XPowder Difdata reference libraries.
FWHM values were calculated using the XPowder software, employing Scherer analysis of width and shape of diffraction profiles modeled on pseudo-Voigt Gaussian curve; measurements for HAP (in the cases where no overlaps are detected) were based on the 2θ = 31.3° peak, while for OCP, these were based on the 2θ = 37.5° peak.
2.5.6 Surface porosity measurements
Surface porosity of samples (pressed bone powder and bone specimens) were measured through SEM Image analysis, by employing ImageJ freeware (ImageJj.net); percent porosity was expressed as the ratio (total area of pores)/(total measured area) × 100. Porosity values were calculated on extracted “analyzed particles” drawing after thresholding the initial image; three different magnifications were examined fir each specimen type. Calculations showed that higher magnifications showed generally higher porosity values due to better image detail; therefore, these values were adopted in the Results section. Values from surface porosity calculations are listed in Table SI.2 (Supplementary Information).
3 Results and discussion
3.1 Experimental design
The precipitation of HAP is a demanding and slow process depending on temperature and ion concentrations because a large number of ions need to be accommodated into the unit crystal. Kinetic studies have shown that a maturing period is needed with intermediate steps involving precursor phosphate phases such as tricalcium phosphate (TCP), brushite, and OCP, before finally forming HAP [1, 67, 75]. In practice, n-HAP and c-HAP, with a generally lower degree of crystallinity than s-HAP, can form; these can be confirmed through their XRD and infrared spectra [31, 58, 67]. A strategy for the synthesis of hydroxyapatite was developed to investigate the factors that control the preferential precipitation of HAP; this involves the reaction of calcium with hydrogen phosphate (HPO42− in DAP) as a source of phosphate ions, as well as SBF and gelatin as biomimetic cofactors.
In the first place, the SBF approach was employed in our methodology as a “training” method for the synthesis of HAP (herewith shown as SBF trials) and used along with DAP and gelatin in trials. Then, to investigate the formation of HAP among other phosphates, a simple methodology involving pressed powder discs of bone powder, plain or calcium-enriched, which serves as the calcium-containing starting material; these were tested against calcium carbonate discs (control experiment) and whole bone specimens (see Materials and Techniques). The discs which serve as mock-ups, allow for (a) deposition of the precipitating product in the intergrain space, (b) controlled geometry for locating the in situ deposited HAP (surface and cross-section), (c) sufficient compactness for investigating the changes in cohesiveness as a means of mechanical endurance, (d) a convenient means for visually assessing the effectiveness of the method and (e) easy application of various analytical methods (ATR-FTIR, SEM–EDX, and finally, XRD) for the detection of products.
This design also allows for investigating bone as the source of calcium, which, in parallel, serves as a biomimetic scaffold for HAP deposition. This combined scenario suggests that some of bone calcium is sacrificed to form carbonated HAP, which, however, affects the integrity of the existing bone. Based on this, additional trials were considered employing CaCl2-pretreated bone powder discs, which leads to calcium-enrichment of the material (BPCa trials). Besides, the effect of SBF and gelatin as possible biomimetic cofactors for the formation of HAP was investigated. In all types of trials, DAP, DAP/SBF, DAP/gelatin, and DASP/SBF/gelatin solutions were studied.
Finally, whole bone specimens were also employed in similar trials to investigate the viability of the method on real objects. In all cases, specimens were water-washed after the reaction, which ensured the removal of all soluble starting materials, as shown through characterization with the selected techniques. The above experimental design, based on previous detailed kinetics studies, focuses on proposing favorable conditions of HAP formation as most suitable for application in the consolidation of bone.
3.2 SBF-based wet synthesis of HAP
Trials with SBF have been employed in the light of previous reports [31, 59, 69] as a wet method for the biomimetic synthesis of HAP. These were, in principle, considered as a chemical process to guide the overall experimental design. Diammonium phosphate (DAP) is the reagent of choice for most research aiming at HAP synthesis so far, as its byproducts are volatile and reportedly works even in lower than physiological temperatures [6, 76] (trials SBF-3 to SBF-6). Additionally, disodium phosphate (DSP) has also been employed further to investigate ionic content (trials SBF-1 and SBF-2).
In addition to the above reagents, gelatin, a biomaterial derived from collagen [77,78,79,80], was also considered as a cofactor since it has been previously reported to promote the formation of HAP crystallites in a favorable molecular geometry [79, 81]. Finally, the trials were performed under stirring, as well as under no stirring, the latter being a condition for demanding applications, such as the conservation of precious heritage artefacts.
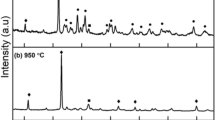
In most cases, results through KBr-FTIR, XRD, and measurements of Ca/P ratios showed that mixtures of HAP with calcium hydrogen phosphate dihydrate (or brushite) and octacalcium phosphate (OCP) were produced (trials SBF-3, and 4). In the case of the unstirred solution of trial SBF-5 with gelatin as a cofactor, HAP and OCP were formed. These two minerals, with expected Ca/P ratios 1.67 and 1.33, respectively, have similar infrared maxima of the prominent v3 and v4 phosphate vibrations [31, 32, 82]. Nevertheless, HAP and OCP can be distinguished through their XRD spectra, as shown in Fig. 2b–d (the reported 2θ angles are relative to CoKa1 wavelength). The d-spacing of H.
AP was found at 3.3210, 2.930, 2.7971, 2.6996, in accordance with reported values [83], those of brushite at 7.5270, 4.1989, 3.0206, 2.9024, 2.5997, 2.4060 and OCP at 3.4061, 2.8041, 2.6122 [31, 84, 85]. The crystal size for brushite (Fig. 2b) was calculated at 35.3 nm (FWHM value calculated for the peak at D-spacing 4.1989 was 0.3070, see Materials and Techniques), while that of HAP (Fig. 2c) at 18.0 nm (FWHM value for the peak at D-spacing 3.3210 was 0.6167). In Fig. 2d, where both products are observed, the exact calculation of crystal size is unclear.
Finally, a carbonate phase is formed in trials SBF-4 and 5 (due to infrared bands at the 1460–1400 cm−1 range and ~ 876 cm−1), possibly favored by the relatively slower diffusion imposed by the applied conditions (unstirred solutions); it is unclear to this point whether it belongs to c-HAP. Selected infrared spectra are shown in Fig. 2a, while peak assignments are listed in Table 2; abbreviated results of trials are summarized in Table 3.
The favorable effect of gelatin for producing the least soluble phases (HAP and OCP) in trial SBF-5 may be partially explained by the relatively higher viscosity induced in the mixing solutions, which under no stirring significantly affected the kinetics of the nucleation of HAP [1, 67, 75]. As brushite and OCP are intermediate phases [33, 86, 87], a role for gelatin in this ionic reaction could be diffusion-controlling, favoring the time-demanding precipitation of the end product. On the other hand, in the trials with disodium phosphate (DSP), almost pure brushite was the product under stirring (trial SBF-1), while HAP with a minor amount of brushite was formed with no stirring (trial SBF-2).
3.3 In situ synthesis of hydroxyapatite on pressed powder discs
The effectiveness of HAP formation during a heterogeneous reaction of DAP in solution with the calcium provided from bone particles was investigated by employing standard 13 mm-discs of pressed bone powder (see Materials and Techniques and Sect. 3.A). The discs offer a convenient means for investigating the course of the reaction in situ on their surface with ATR-FTIR spectroscopy, as well as investigating the micro-morphology of the products with SEM.
3.3.1 Pressed CaCO3 powder discs (control trials)
The hypothesis that bone components promote HAP formation by serving as a biomimetic scaffold and simultaneously providing calcium ions to react with externally provided phosphate was evaluated and compared to control trials on pressed CaCO3 (calcite) discs. In these, calcite is the Ca2+ source, while biomimetic conditions are offered from cofactors SBF and gelatin. The porosity of the initial state of specimens, a crucial factor that allows for product nucleation, was measured through SEM image analysis [88,89,90] (see Materials and Techniques). In pressed powder disc-based trials, surface porosity can be defined as the gaps between grain boundaries, which can be related to the cohesiveness of grains of the pressed disc. In the case of pressed CaCO3 discs, surface porosity was found to be 25–30% (see Table SI.2, Supplementary Information).
In trials Ca-1, 2, 3, and 4, the immersion of pressed CaCO3 powder disks in DAP solutions (see Materials and Techniques) generally resulted in deposited material composed of HAP and brushite mixtures as identified through their Ca/P ratios, and their ATR-FTIR and XRD spectra (Fig. 3 and Table 3). The reaction of calcite with DAP has been previously studied by atomic force microscopy (AFM), and the formation of carbonate hydroxyapatite was reported, although no further information was provided [76].
As investigated through the combined spectroscopic and elemental analysis techniques, DAP and DAP/collagen solutions (trials Ca-1, 2, and 3) resulted in brushite/HAP mixtures, while DAP/SBF/gelatin (Ca-4) resulted in a product mixture rich in HAP. Infrared spectra of products after removal of soluble components (reactants) are shown in Fig. 3. Notably, the synergistic action of gelatin with all other reagents promotes the predominant nucleation of HAP. Biomacromolecules such as gelatin allow a favorable alignment for the nucleation of HAP [30, 91], although aminoacids have been reported to inhibit HAP crystallization [92, 93], suggesting a complex multi-site mechanism concerning molecular geometries [30]. The result in the present work confirms previous findings and suggests that gelatin may be considered as a biomimetic cofactor in these applications.
Interestingly, further addition of the above reagents to the calcite powder discs (trials not shown) increased the brushite amount significantly (see, for instance, spectrum v, in Fig. 3, which shows the product of trial Ca-4 after further addition of reagents). Scanning Electron Microscopy (SEM) images are shown in Supplementary information (Figure SI.1a), where newly formed crystal aggregates are shown as compared to reference bone.
These results also suggest possible conditions for the optimized precipitation of HAP on the surface of inorganic substrates such as limestone and marble, which have attracted significant attention in the conservation of antiquities [4, 6, 94, 95].
3.3.2 Pressed bone powder disks (trials BP)
Bone powder discs (see Materials and Techniques) offer a convenient medium for in situ evaluating the product formation with ATR-FTIR and SEM–EDX after the completion of reactions. Previous work has shown that in situ deposited HAP can offer consolidation to bone by reducing the porosity of (non-pressed) bone powder [7]; therefore, since HAP is precipitated in the inter-grain area of a pressed bone powder disc by similarly reducing its porosity, strengthening may be offered to the disc. Moreover, the pressing of grains during disc preparation results in cohesion for holding the hydraulically pressed disc. Surface porosities, as measured through SEM image analysis, were found to be 10.7–13.9% (see Materials and Techniques, Table SI.2).
Interestingly, a single drop of deionized water on the bone powder disc surface manages to completely disrupt the disc by separating the grains from each other.Footnote 1 Based on the above, on an experimental basis, any improvement regarding the cohesiveness of discs after HAP-forming trials can be an indication for potential consolidation offered by the deposited product. However, by visual observation (photos in insets of Fig. 4a), discs were significantly damaged, except for the trial with DAP/SBF (BP-2) where the disc integrity was preserved; water washup for removing all soluble material did not further change the condition of the disc.
The Ca/P ratios were found to be close to those of reference bone (Ca/P = 1.37, Table 3), although they cannot be solely used as a criterion [96, 97]. FTIR spectra of trial BP-1 showed an increase in both phosphate and carbonate type A and B peaks, suggesting that c-HAP is formed; on the other hand, in trials (BP-2, 3, and 4), the product quantities are negligible, as compared with the initial bone spectrum (Fig. 4a). For the sake of comparison, all spectra are normalized in the amide I band of collagen. Elemental microanalysis showed minimal chlorine content after water washup, an indication first, that soluble material is removed, and secondly, that chlorapatite is not a possible product. These results suggest that calcium provided by bone powder is possibly not sufficient to ensure the integrity of the disk by forming HAP.
SEM microphotographs (Supplementary Information, Figure SI.1b) support this, by not clearly showing new crystals formation on the specimen surface, except for case BP-1 (Fig. 5b).
a Photomicrographs of products after trials; a SEM of Ca-enriched bone powder discs (trials BPCa-1 and BPCa-4); b SEM of bone specimens (trials B-1 and B-4); (0) calcite disc before addition of reagents; (i) after immersion in DAP, (ii) after immersion in DAP/SBF/gelatin solution. c TEM image of products on bone specimens (trials B-1 and B-4); after immersion in (i) DAP, (ii) DAP/SBF/gelatin solution
3.3.3 Pressed calcium-enriched bone powder disks (trials BPCa)
Similar trials (BPCa-1, 2, 3, and 4) were done on calcium-enriched disks (see Materials and Techniques), ensuring that bone material would not be deprived of its calcium as the enrichment process would be the primary Ca2+ source. Calcium chloride was chosen because it could be easily removed with water-washup at the end of the process, and it would not interfere with the pH of the process, as well as with the monitoring of the entire process through infrared spectroscopy. The removal of CaCl2 after washup was tested through microelemental analysis where, in all cases, chlorine was found less than 0.9% atom (1.43 wt%).
SEM microphotographs show the deposition of the crystalline product (crystal size 10–20 μm) on the surface of specimens (Fig. 5c). The Ca/P ratios of products were found between 1.35 and 1.41, close to those of reference bone (1.37).
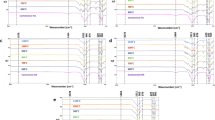
The XRD spectra of trials BPCa-1 and 2 showed the formation of HAP (Fig. 6a and b), while BPCa-3 and 4 showed mixtures of HAP with OCP (Fig. 6c and d); D-spacings are shown as figures alongside peaks. HAP was found to have larger crystals as calculated through line shape analysis of XRD peaks (see Materials and Techniques); FWHM values ranged from 0.7481 (corresponding to 15.3 nm crystal size, trial PBCa-1) to 0. 0862 (123.4 nm, trial BPCa-2). By comparison, the OCP-containing spectra (Fig. 6iii and iv) were broader, corresponding to significantly smaller crystals; FWHM was found to be 1.8934 (corresponding to 5.9 nm, trial BPCa-3) and 1.8103 (6.2 nm, trial BPCa-4).
The infrared spectra (Fig. 4b) showed the HAP profile [31, 52, 53]; in particular, the IRSF values of the v4 phosphate vibration in infrared spectra generally show higher crystallinities than those of bone reference, especially for trial BPCa-1 (see Table SI.1). Moreover, the ATR-FTIR spectra recorded on the discs showed increased phosphate phases (based on the maxima of v3 and v4 vibrational modes, Fig. 4b). In particular, the DAP/SBF reagent mixture (trial BPCa-2) gave the highest amount of HAP, seen through its prominent peaks at ~ 1020 cm−1 and the doublet at 601/558 cm−1 in Fig. 4b (i) (see Table 2 for peak assignments). On the other hand, the DAP/gelatin trial (BPCa-3) showed infrared shoulders at 1160, 1104, 1054, 916 cm−1, assignable to OCP [30, 31, 82]. Carbonate peaks of type A (1455 cm−1) and B (1410 cm−1) were detected in the product of trials BPCa-1, 2, and 4 (Fig. 4b). The carbonate index was generally lower as compared to reference bone (see Table SI1); specifically, that of trial BPCa-1 was exceptionally low. This result shows that DAP alone favors the formation of n-HAP instead of c-HAP. On the other hand, trial BPCa-3 resulted in labile calcium carbonate as detected through its uniform, unresolved v3 maximum at ~ 1413 cm−1.
The crystallinity index, expressed through the infrared splitting factor (IRSF, see Introduction) [11, 74, 84], was found ~ 10–12% higher than that of fresh bone (see values in Table SI.1). HAP was found to be produced at almost six-fold excess over that of reference bone (estimated through the collagen index, i.e., the ratio of the collagen amide I absorbance height at 1660 cm−1 over that of phosphate v3 at 1020 cm−1 [49, 55] (see Table SI1).
Through the trials on bone powder discs, the dropwise addition of reactants does not allow for long reaction times, thus restricting the observed phenomenon to its early stages. According to Ostwald’s rules, more soluble, although less stable, precursor phases, such as OCP, are formed with small crystal size due to relatively fast nucleation [6, 91, 92]; these are followed by a slower nucleation stage, where HAP is formed. These experiments observe a time window where HAP starts to nucleate. For consolidation purposes, both products could work due to their comparably low solubility.
Significantly, the integrity of discs is mostly preserved in all Ca-enriched trials (insets of Fig. 4b). By comparing this observation with that of bone powder discs of trials BP1 to BP4, it seems that the extra calcium deposited on the bone grains through enrichment may be responsible for significant HAP deposition on the disks that allow better cohesion of the material.
3.4 Whole bone specimens
The in situ synthesis of HAP was also tested on the surface of dead bird bone specimens after immersion trials in DAP-containing solutions (see Materials and Techniques), additionally containing gelatin, SBF, and their combinations. The average surface porosity of bone was found to be approximately 15%. However, as its surface is highly inhomogeneous in certain spots, such as surface grooves, high magnifications (3000 × and 5000 × ) showed porosity to be as high as 21%.
The deposition of white crystalline material on the bone surface was observed in all cases. After washing up the specimen surfaces with deionized water, the detached product was studied with SEM–EDX and FTIR spectroscopy. In all cases, washing up removed most soluble reactants and possible byproducts, leaving the least soluble components of the products. SEM microphotographs show product deposition on the surface of bone (Fig. 5c, photos (i–iv)) in comparison with the surface of reference (untreated) bone (photo (0), same Figure). Elemental microanalysis of the produced crystals with SEM–EDX resulted in Ca/P ratios (Table 3) of approx. 1.26, while the B-1 trial, specifically, gave 1.64, which is close to the expected ratio for s-HAP.
Transmission Electron Microscopy (TEM) bright-field images for trial B-1 (Fig. 5d) showed approx. 100–120 nm needle-like nHAP crystals [2, 57] formed close to smaller (< 50 nm) original bone mineral crystals, while EDX analysis showed that elemental Ca/P ratios ranged between 1.41–1.63. TEM image for the B-4 trial also showed nHAP together with smaller original bone crystals, as well as a surrounding amorphous material, possibly gelatin.
The quantities of the final insoluble products did not allow for conclusive XRD spectra, as no detectable phases were measured. The FTIR spectra showed increased phosphate (v3 and v4 bands) due to the formation of HAP (Fig. 7); this increase is more significant in trial B-4, due to a combined effect of the three reagents. Although the carbonate peaks generally increase as compared to collagen amide I, the carbonate index is comparably lower than that of the original bone (see Table SI1). These results suggest that although both n-HAP and c-HAP are formed, the former is preferred to the latter. The crystallinity index (IRSF value) of the product was increased by 10–12% as compared to that of reference bone (Table SI1). Moreover, deconvolution of the v4 phosphate band (Fig. 7b and c for reference bone and product of trial B-4, respectively) shows the contributions of apatitic PO43− (at ~ 601, 575, and 560 cm−1) and HPO43− peaks (apatitic at ~ 545 cm−1, and non-apatitic at ~ 615 and 530 cm−1) [98]. In spectrum 7c, the higher-than-expected peaks at 578 and 541 cm−1 are possibly contributions from the v4 vibrations of co-produced OCP (cf. with values in Table 2).
a ATR-FTIR spectra from Ca-enriched bone specimens after trials B-1, 2, 3 and 4: (0) reference bone; product formation on the bone surface after immersion (i) in DAP (trial B-1), (ii) in DAP/SBF (trial B-2), (iii) in DAP/gelatin (trial B-3) and (iv) in DAP/SBF/gelatin (trial B-4) solutions. All spectra were recorded after the removal of starting material by water washup. Deconvolution and peak fitting of the phosphate v4 vibration in b reference bone (spectrum a0); c product after trial B-1
The adopted experimental design and the results of this work helped in isolating factors, such as the substrate nature, the surface morphology, as well as the protein and ionic content in the molecular environment. As a significant outcome, collagen is confirmed to have a favoring effect in the nucleation of apatitic phases. Based on the hypothesis of a macromolecule-stabilized liquid phase [99], the macromolecular structure of collagen with its specific aminoacid content and sequence serves as a favorable template for the nucleation of phosphates by aligning the c-axes of apatitic crystals with the direction of collagen molecule [81, 100]. With a hydration sphere that favors the clustering of calcium and phosphate ions due to mutual ionic attractions, a favorable ionic profile (SBF) along with the collagen or gelatin macromolecular template promotes precursor phases, such as brushite and OCP [91, 101]. The above is an early stage that eventually leads to slower, gradual nucleation of the thermodynamically more stable and relatively larger HAP crystals. The mediation of bone collagen, which in these experiments was assisted by externally added gelatin and SBF, signifies the biomimetic character of the process.
Although maturing time is needed for sufficient HAP formation [30], the co-precipitation of OCP in some cases could be considered not a problem for consolidation purposes, as this mineral also has a low Ksp value (Table 1).
4 Conclusions
This work shows that hydroxyapatite is biomimetically nucleated on the calcium-rich surfaces of pressed bone powder discs and whole bone specimens, by employing a soluble phosphate (DAP) and cofactors such as SBF and gelatin. Bone works as a biomimetic scaffold, which, aided by externally added gelatin, and SBF directs calcium, phosphate, and eventually carbonate ions to nucleate into carbonate hydroxyapatite (c-HAP), along with precursor phases, such as octacalcium phosphate (OCP). In cases where reaction times were long enough (whole bone specimens), hydroxyapatite was predominantly detected. Interestingly, the combined action of SBF and gelatin favored the formation of c-HAP even in the absence of bone scaffold, as findings from the controlling experiments on calcite discs suggest. In the case of trials with calcium-enriched specimens, sacrificial consumption of bone calcium was avoided, and the integrity of the disks was mostly unaffected, suggesting mechanical endurance of the resulting specimens; furthermore, the favorable role of gelatin was highlighted.
The results show that this strategy is promising for the consolidation of deteriorated bone. At the same time, they encourage for further optimization by exploring the gelatin factor, by replacing calcium chloride by calcium hydroxide, and by controlling diffusion and selectivity with the use of gelling agents. Moreover, a specific design for mechanical testing is needed, where the practical scope of this method will be ensured.
Notes
For comparison, the CaCO3 discs in the control trials had maintained their integrity upon the addition of water and all other reagent solutions, as well (not shown).
Abbreviations
- ATR:
-
Attenuated total reflection
- DAP:
-
Diammonium phosphate, (NH4)2HPO4,
- DSP:
-
Disodium phosphate, Na2HPO4,
- EDX:
-
Energy Dispersive X-ray,
- HAP:
-
Hydroxyapatite
- c-HAP:
-
Carbonate hydroxyapatite
- n-HAP:
-
Nanocrystalline hydroxyapatite
- s-HAP:
-
Stoichiometric hydroxyapatite
- FTIR:
-
Fourier transform infrared spectroscopy
- IRSF:
-
Infrared Splitting Factor
- SBF:
-
Simulated Body Fluid
- SI:
-
Supplementary Information
- SEM:
-
Scanning Electron Microscopy,
- XRD:
-
X-ray diffractometry
References
Nayak AK (2010) Hydroxyapatite synthesis methodologies: an overview. Int J ChemTech Res 2:903–907
Roveri N, Foresti E, Lelli M et al (2010) Microscopic investigations of synthetic biomimetic hydroxyapatite. In: Méndez-Vilas A, Díaz J (eds) Microscopy: science, technology, applications and education, microscopy series N° 4, vol 3. Formatex Research Cente, pp 1868–1879
Matteini M (2008) Inorganic treatments for the consolidation and protection of stone artefacts and mural paintings. Conserv Sci Cult Herit 8:13–27
Sassoni E, Naidu S, Scherer GW (2011) The use of hydroxyapatite as a new inorganic consolidant for damaged carbonate stones. J Cult Herit 12:346–355
Scherer GW, Wheeler GS (2008) Silicate Consolidants for Stone. Key Eng Mater 391:1–25
Sassoni E (2018) Hydroxyapatite and other calcium phosphates for the conservation of cultural heritage: a review. Materials 11:557
North A, Balonis M, Kakoulli I (2016) Biomimetic hydroxyapatite as a new consolidating agent for archaeological bone. Stud Conserv 61:146–161
Weiner S, Wagner HD (1998) The material bone: structure-mechanical function relations. Annu Rev Mater Sci 28:271–298
Weiner S (2010) Microarchaeology, 1st edn. Cambridge University Press, Cambridge
Leventouri T (2006) Synthetic and biological hydroxyapatites: crystal structure questions. Biomaterials 27:3339–3342
Miller LM, Vairavamurthy V, Chance MR et al (2001) In situ analysis of mineral content and crystallinity in bone using infrared micro-spectroscopy of the v4 PO4(3-) vibration. Biochim Biophys Acta 1527:11–19
Qin Z, Gautieri A, Nair AK et al (2012) Thickness of hydroxyapatite nanocrystal controls mechanical properties of the collagen-hydroxyapatite interface. Langmuir 28:1982–1992
Surovell TA, Stiner MC (2001) Standardizing infra-red measures of bone mineral crystallinity: an experimental approach. J Archaeol Sci 28:633–642
Lee-Thorp J (2002) Two decades of progress towards understanding fossilization processes and isotopic signals in calcified tissue minerals. Archaeometry 44:435–446
Kendall C, Eriksen AMH, Kontopoulos I et al (2018) Diagenesis of archaeological bone and tooth. Palaeogeogr Palaeoclimatol Palaeoecol 491:21–37
Nielsen-Marsh CM, Hedges REM (2000) Patterns of diagenesis in bone II: effects of acetic acid treatment and the removal of diagenetic CO32−. J Archaeol Sci 27:1151–1159
Hedges REM (2002) Bone diagenesis: an overview of processes. Archaeometry 44:319–328
Mays S (1998) The archaeology of human bones. Am Anthropol 102:175–176
Collins MJ, Riley MS, Child AM, Turner-Walker G (1995) A basic mathematical simulation of the chemical degradation of ancient collagen. J Archaeol Sci 22:175–183
Maksoud GA (2010) Comparison between the properties of ʺaccelerated-agedʺ bones and archaeological bones. Mediterr Archaeol Archaeom 10:89–112
Grunenwald A, Keyser C, Sautereau AM et al (2014) Novel contribution on the diagenetic physicochemical features of bone and teeth minerals, as substrates for ancient DNA typing. Anal Bioanal Chem 406:4691–4704
Johnson JS (1994) Consolidation of archaeological bone: a conservation perspective. J F Archaeol 21:221–233
Yang F, He D, Liu Y et al (2016) Conservation of bone relics using hydroxyapatite as protective material. Appl Phys A 122:479
Dorozhkin SV (2011) Calcium orthophosphates. Biomatter 1:121–164
Wopenka B, Pasteris JD (2005) A mineralogical perspective on the apatite in bone. Mater Sci Eng C 25:131–143
Hafiz M, Matsumoto T, Okazaki M et al (2010) Biomimetic Fabrication of Apatite Related Biomaterials. In: Mukherjee A (ed) Biomimetics learning from nature. IntechOpen
Raucci MG, Guarino V, Ambrosio L (2012) Biomimetic strategies for bone repair and regeneration. J Funct Biomater 3:688–705
Palmer LC, Newcomb CJ, Kaltz SR et al (2008) Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem Rev 108:4754–4783
He W, Kjellin P, Currie F et al (2012) Formation of bone-like nanocrystalline apatite using self-assembled liquid crystals. Chem Mater 24:892–902
Gómez-Morales J, Michele I, Delgado-López JM et al (2013) Progress on the preparation of nanocrystalline apatites and surface characterization: overview of fundamental and applied aspects. Prog Cryst Growth Charact Mater 59:1–46
Drouet C (2013) Apatite formation: why it may not work as planned, and how to conclusively identify apatite compounds. Biomed Res Int 2013:1–12
Rey C, Shimizu M, Collins B, Glimcher MJ (1990) Resolution-enhanced fourier transform infrared spectroscopy study of the environment of phosphate ions in the early deposits of a solid phase of calcium-phosphate in bone and enamel, and their evolution with age. I: investigations in the v4 PO4 domain. Calcif Tissue Int 46:384–394
LeGeros R, Daculsi G, Orly I et al (1989) Solution-mediated transformation of octacalcium phosphate (OCP) to apatite. Scanning Microsc 3:129–137
Berna F, Matthews A, Weiner S (2004) Solubilities of bone mineral from archaeological sites: the recrystallization window. J Archaeol Sci 31:867–882
Stiner MC, Kuhn SL, Surovell TA et al (2001) Bone preservation in hayonim cave (Israel): a macroscopic and mineralogical study. J Archaeol Sci 28:643–659
Weiner S, Goldberg P, Bar-Yosef O (1993) Bone preservation in Kebara Cave, Israel using on-site fourier transform infrared spectrometry. J Archaeol Sci 20:613–627
Weiner S, Bar-Yosef O (1990) States of preservation of bones from prehistoric sites in the near east: a survey. J Archaeol Sci 17:187–196
Kasem MA, Yousef I, Alrowaili ZA et al (2020) Investigating Egyptian archeological bone diagenesis using ATR-FTIR microspectroscopy. J Radiat Res Appl Sci 13:515–527
Bayarı SH, Özdemir K, Sen EH et al (2020) Application of ATR-FTIR spectroscopy and chemometrics for the discrimination of human bone remains from different archaeological sites in Turkey. Spectrochim Acta Part A Mol Biomol Spectrosc 237:118311
Ulian G, Valdre G, Corno M, Ugliengo P (2013) The vibrational features of hydroxylapatite and type a carbonated apatite: a first principle contribution. Am Mineral 98:752–759
Langford JI, Wilson AJC (1978) Scherrer after sixty years: a survey and some new results in the determination of crystallite size. J Appl Crystallogr 11:102–113
Muhammed Shafi P, Chandra Bose A (2015) Impact of crystalline defects and size on X-ray line broadening: a phenomenological approach for tetragonal SnO 2 nanocrystals. AIP Adv 5:057137
Zhu Y, Xu L, Liu C et al (2018) Nucleation and growth of hydroxyapatite nanocrystals by hydrothermal method. AIP Adv 8:085221
Stutman JM, Termine JD, Posner AS (1965) Vibrational spectra and structure of the phosphate ion in some calcium phosphates*,†. Trans N Y Acad Sci 27:669–675
Nakamoto K (2006) Infrared and Raman spectra of inorganic and coordination compounds. In: Chalmers JM, Griffiths PR (eds) Handbook of vibrational spectroscopy. Wiley, Chichester
Grynpas M (1976) The crystallinity of bone mineral. J Mater Sci 11:1691–1696
Miller LM, Vairavamurthy V, Chance MR et al (2001) In situ analysis of mineral content and crystallinity in bone using infrared micro-spectroscopy of the v(4) PO43- vibration. Biochim Biophys Acta-General Subj 1527:11–19
Brundavanam RK, Eddy G, Poinern J, Fawcett D (2013) Modelling the crystal structure of a 30 nm sized particle based hydroxyapatite powder synthesised under the influence of ultrasound irradiation from X-ray powder diffraction data. Am J Mater Sci 3:84–90
Dal Sasso G, Asscher Y, Angelini I et al (2018) A universal curve of apatite crystallinity for the assessment of bone integrity and preservation. Sci Rep 8:1–13
El Feki H, Rey C, Vignoles M (1991) Carbonate ions in apatites: Infrared investigations in the v4 CO3 domain. Calcif Tissue Int 49:269–274
Fleet ME, Liu X, King PL (2004) Accommodation of the carbonate ion in apatite: an FTIR and X-ray structure study of crystals synthesized at 2–4 GPa. Am Miner 89:1422–1432
Fleet ME (2009) Infrared spectra of carbonate apatites: ν2-region bands. Biomaterials 30:1473–1481
Fleet ME (2017) Infrared spectra of carbonate apatites: evidence for a connection between bone mineral and body fluids. Am Mineral 102:149–157
Ou-Yang H, Paschalis EP, Mayo WE et al (2001) Infrared microscopic imaging of bone: spatial distribution of CO3(2-). J Bone Miner Res 16:893–900
Chadefaux C, Le Hô A-S, Bellot-Gurlet L et al (2009) Curve-fitting Micro-ATR-FTIR studies of the amide I and II bands of type I collagen in archaeological bone materials. e-PS 6:129–137
Grunenwald A, Keyser C, Sautereau AM et al (2014) Revisiting carbonate quantification in apatite (bio)minerals: a validated FTIR methodology. J Archaeol Sci 49:134–141
Koutsopoulos S (2002) Synthesis and characterization of hydroxyapatite crystals: a review study on the analytical methods. J Biomed Mater Res 62:600–612
Ohtsuki C (2009) How to prepare the simulated body fluid (SBF) and its related solutions, proposed by Kokubo and his colleagues. Grad Sch Mater Sci Nara Inst 3:5–8
Kokubo T, Kushitani H, Sakka S et al (1990) Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J Biomed Mater Res 24:721–734
Pecheva E, Pramatarova L (2006) Modified Inorganic Surfaces as a Model for Hydroxyapatite Growth. Trans Tech Publications Ltd, Switzerland, Uetikon-Zuerich
Kien PT, Phu HD, Linh NVV et al (2018) Recent trends in hydroxyapatite (HA) synthesis and the synthesis report of nanostructure HA by hydrothermal reaction. In: Chun HJ, Park K, Kim C-H, Khang G (eds) Novel biomaterials for regenerative medicine. Springer, Singapore, pp 343–354
Shin K, Acri T, Geary S, Salem AK (2017) Biomimetic mineralization of biomaterials using simulated body fluids for bone tissue engineering and regenerative medicine. Tissue Eng Part A 23:1169–1180
Nweke CE, Stegemann JP (2020) Modular microcarrier technologies for cell-based bone regeneration. J Mater Chem B 8:3972–3984
Xu G, Aksay IA, Groves JT (2001) Continuous crystalline carbonate apatite thin films. a biomimetic approach. J Am Chem Soc 123:2196–2203
Vallet-Regi M, Navarrete DA (2015) Nanoceramics in clinical use. Royal Society of Chemistry, Cambridge
Arcos D, Vallet-Regí M (2020) Substituted hydroxyapatite coatings of bone implants. J Mater Chem B 8:1781–1800
Cuneyt Tas A (2000) Synthesis of biomimetic Ca-hydroxyapatite powders at 37°C in synthetic body fluids. Biomaterials 21:1429–1438
Kokubo T, Takadama H (2006) How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 27:2907–2915
Oyane A, Kim H-M, Furuya T et al (2003) Preparation and assessment of revised simulated body fluids. J Biomed Mater Res 65A:188–195
Kim H-M, Kishimoto K, Miyaji F et al (1999) Composition and structure of the apatite formed on PET substrates in SBF modified with various ionic activity products. J Biomed Mater Res 46:228–235
Zhou Q, Su C-Y, Zheng J-J (2020) Photocatalytic HA deposition on TiO2 of Ti-0.2Pd surface immersed in simulated body fluid. Surf Coatings Technol 389:125649
Hollund HI, Ariese F, Fernandes R et al (2013) Testing an alternative high-throughput tool for investigating bone diagenesis: FTIR in attenuated total reflection (ATR) mode. Archaeometry 55:507–532
Kontopoulos I, Presslee S, Penkman K, Collins MJ (2018) Preparation of bone powder for FTIR-ATR analysis: the particle size effect. Vib Spectrosc 99:167–177
Tătar A, Ponta O, Kelemen B (2014) Bone diagenesis and ftir indices: a correlation. Stud Univ Babeş Bolyai Biol 59:101–113
Lin X, Li X, Fan H et al (2004) In situ synthesis of bone-like apatite/collagen nano-composite at low temperature. Mater Lett 58:3569–3572
Kamiya M, Hatta J, Shimada E et al (2004) AFM analysis of initial stage of reaction between calcite and phosphate. Mater Sci Eng B Solid-State Mater Adv Technol 111:226–231
Eastoe JE (1957) The amino acid composition of fish collagen and gelatin. Biochem J 65:363–368
Yakimets I, Wellner N, Smith AC et al (2005) Mechanical properties with respect to water content of gelatin films in glassy state. Polymer (Guildf) 46:12577–12585
Zandi M (2008) Studies on the gelation of gelatin solutions and on the use of resulting gels for medical scaffolds. Duisburg-Essen
Gorgieva S, Kokol V (2011) Collagen- vs. Gelatine-based biomaterials and their biocompatibility: review and perspectives. In: Pignatello R (ed) Biomaterials applications for nanomedicine. InTech, pp 1–36
Bigi A, Boanini E, Panzavolta S, Roveri N (2000) Biomimetic growth of hydroxyapatite on gelatin films doped with sodium polyacrylate. Biomacromol 1:752–756
Fowler BO, Marković M, Brown WE (1993) Octacalcium phosphate. 3. Infrared and Raman vibrational spectra. Chem Mater 5:1417–1423
Cullity BD, Stock SR (2013) Elements of X-ray diffraction, 3rd edn. Pearson New International Edition, Edinbourgh Gate
Reyes-Gasga J, Martínez-Piñeiro EL, Rodríguez-Álvarez G et al (2013) XRD and FTIR crystallinity indices in sound human tooth enamel and synthetic hydroxyapatite. Mater Sci Eng C 33:4568–4574
Nilen RWN, Richter PW (2008) The thermal stability of hydroxyapatite in biphasic calcium phosphate ceramics. J Mater Sci Mater Med 19:1693–1702
Zapanta Le Geros R (1974) Variations in the crystalline components of human dental calculus: I. Crystallographic and spectroscopic methods of analysis. J Dent Res 53:45–50
Mróz W, Bombalska A, Budner B et al (2010) Comparative study of hydroxyapatite and octacalcium phosphate coatings deposited on metallic implants by PLD method. Appl Phys A Mater Sci Process 101:713–716
Elsen J, Groot C (2007) Characterisation: porosity of mortars. In: Groot C, Ashall G, Hughes J (eds) Characterisation of old mortars with respect to their repair. RILEM publications SARL, Paris
Beningfield N, Winter N, Farrell S (2012) An interim investigation into pore formation in masonry mortar and the use of microscopy to investigate the probable initial air content. Mason Int 25:9–18
Shawnim P, Mohammad F (2019) Porosity, permeability and microstructure of foamed concrete through SEM images. J Civ Eng Sci Technol 10:22–33
Ethirajan A, Ziener U, Chuvilin A et al (2008) Biomimetic hydroxyapatite crystallization in gelatin nanoparticles synthesized using a miniemulsion process. Adv Funct Mater 18:2221–2227
Koutsopoulos S, Dalas E (2000) The crystallization of hydroxyapatite in the presence of lysine. J Colloid Interface Sci 231:207–212
Koutsopoulos S, Dalas E (2000) Hydroxyapatite crystallization in the presence of serine, tyrosine and hydroxyproline amino acids with polar side groups. J Cryst Growth 216:443–449
Possenti E, Colombo C, Bersani D et al (2016) New insight on the interaction of diammonium hydrogenphosphate conservation treatment with carbonatic substrates: A multi-analytical approach. Microchem J 127:79–86
Ma X (2017) In situ Synthesis and Characterizations of Bio-ceramic Based Hydroxyapatite in the Re-mineralization of Calcium-rich Matrices (PhD Thesis). UCLA
Sotiropoulou P, Fountos G, Martini N et al (2015) Bone calcium/phosphorus ratio determination using dual energy X-ray method. Phys Med 31:307–313
Kourkoumelis N, Balatsoukas I, Tzaphlidou M (2012) Ca/P concentration ratio at different sites of normal and osteoporotic rabbit bones evaluated by Auger and energy dispersive X-ray spectroscopy. J Biol Phys 38:279–291
Vandecandelaere N, Rey C, Drouet C (2012) Biomimetic apatite-based biomaterials: on the critical impact of synthesis and post-synthesis parameters. J Mater Sci Mater Med 23:2593–2606
Rao A, Cölfen H (2018) From solute, fluidic and particulate precursors to complex organizations of matter. Chem Rec 18:1203–1221
Ichikawa R, Kajiyama S, Iimura M, Kato T (2019) Tuning the c-axis orientation of calcium phosphate hybrid thin films using polymer templates. Langmuir 35:4077–4084
Almora Barrios N (2010) A computational investigation of the interaction of the collagen molecule with hydroxyapatite. University College London
Acknowledgements
The authors wish to thank: Dr. Jane Richter, Associate Professor at the Royal Danish Academy of Fine Arts (DRAFA), School of Conservation, Copenhagen, Denmark for supplying with Roe deer bone used in powder discs; Mrs. Eleni Tziamourani for technical assistance with grinding bone into powder; Mr. Athanasios Karampotsos, conservator, for supervision and technical assistance in the SEM-EDX facility of the University of West Attica) and Dr. Alexis Stefanis (Assistant Professor, University of West Attica) for their valuable help on experimental procedures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standard
No animals were tortured or sacrificed for the sample needs of this work. Roe deer bone samples were obtained through a slaughterhouse in Denmark. Naturally-aged dead seagull bone was collected after it was found in the open air.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nesseri, E., Boyatzis, S.C., Boukos, N. et al. Optimizing the biomimetic synthesis of hydroxyapatite for the consolidation of bone using diammonium phosphate, simulated body fluid, and gelatin. SN Appl. Sci. 2, 1892 (2020). https://doi.org/10.1007/s42452-020-03547-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-03547-8