Abstract
Dark fermentation course analysis is crucial, as complexed matrix of gaseous components may be formed and revealed during the process. The paper considers key issues related to the microbiological process in which complex organic substances are transformed into hydrogen. For the purposes of hydrogen generation, the application of wastewater mixed sludge pre-treated according to Faloye method (Faloye et al. in Int J Hydrog Energy 38:11765–11773, 2013. https://doi.org/10.1016/j.ijhydene.2013.06.129; Int J Hydrog Energy 39:5607–5616, 2014. https://doi.org/10.1016/j.ijhydene.2014.01.163) was applied. The main risk of by-product formation is related to the presence of methanogens, i.e., Archea, in the sludge. The application of gaseous chromatography confirmed the presence of hydrogen during the initial, lag and log phases of the culture and methane in the late logarithmic death phase of the culture. However, other fermentation gaseous products’ presence was not confirmed, as their concentration was under the limit of detection. Therefore, a revision regarding the application of matrix sensors was proposed, and the levels of gases able to be measured using both gas chromatography and matrix sensors were conducted. The criteria of matrix sensors’ selection should include the selectivity not only for the hydrogen, hydrogen sulfide or methane, but also the sensitivity to the response of other gases contained in the mixture—ammonium, carbon dioxide and oxygen. A comprehensive combination of commercially available sensors and their applicability for the purposes of dark fermentation course analysis was presented on the basis of the levels of gas concentrations in the generated gas mixture.
Similar content being viewed by others
1 Introduction
Fermentation is a group of biological process of decomposition of organic substances leading to stabilization of sewage sludge, waste materials and residues. Diversified phenomena occur during fermentation processes, as a result of the entire range of organic compounds degradation with the formation of, that is, methane, ethanol or hydrogen and carbon dioxide [3,4,5]. Currently, fermentation is used in four main sectors of waste processing including treatment of urban wastewater, agricultural waste, food and biomass industries and processing of the organic fraction of municipal solid waste [5,6,7]. Biomass residues of wide origin are considered as great potential materials for biofuels production [3, 8, 9]. The main directions in biomass to biofuels transformation are presented in Fig. 1.
The objective of this paper is the research on dark fermentation to biohydrogen and the factors affecting this process. Hydrogen is the most widespread element in nature. Non-renewable fossil raw materials such as crude oil (about 50%), natural gas (30%) and coal (15%) [10, 11] are the most commonly used sources for hydrogen production. Among the leading technologies for hydrogen production using conventional energy sources are steam reforming of natural gas and crude oil, the catalytic decomposition of natural gas, partial oxidation of heavy hydrocarbon fractions of crude oil and gasification of coal or coke. Unfortunately, these methods are highly energy-intensive, require the use of high temperatures (> 700 °C) and pollute the environment due to the carbon, sulfur and nitrogen oxides emission [12, 13]. Another important industrial process for the production of hydrogen is the electrolysis of water, under the influence of electric current. This method, however, requires delivery of electricity from coal and natural gas or nuclear power plants. Therefore, non-renewable fossil fuels are used indirectly as raw materials. It is estimated that the cost of electricity represents about 80% of the total cost of the process [14], and the production of hydrogen by electrolysis of water is currently about 3.5 times more expensive compared to natural gas steam reforming [15]. The advantage of electrolysis is, however, no emission of carbon dioxide into the atmosphere. Other hydrogen production methods, such as thermochemical or photocatalytic decomposition of water and a high-temperature plasma technology separation of hydrocarbons into hydrogen and carbon, are the subject of intensive research into their development.
Hydrogen conversion from biomass may be a good alternative if biotechnological methods are used. The biological technologies of producing hydrogen include processes involving light energy: direct and indirect biophotolysis and photofermentation. The second group are the biological processes in the absence of light: dark fermentation, bioelectrolysis and bioconversion of carbon monoxide [16]. The dark fermentation is considered as the most promising method for biological hydrogen production. Carbohydrate substrates are converted in the absence of light by bacteria in the process of anaerobic respiration. The raw materials for the fermentation may be glycerin or simple sugars as well as cellulose or starch, which can be hydrolyzed to monosaccharides [17, 18]. In particular, it is desirable to use by-products, waste products rich in glycerol, starch and cellulose from agri-food, wood and paper industries to produce the second-generation biohydrogen using microbial metabolic processes. While the biomass contains high amounts of polysaccharides, which can be processed into hydrogen, it be an alternative to currently used processes, such as combustion or composting. Utilization of waste products that are difficult to dispose can contribute to environmental protection through both more rational use of natural resources and reducing the environmental load of such waste.
The first step in dark fermentation is glycolysis—glucose is fermented to pyruvate. Pyruvate is oxidized and transfers the electrons to ferredoxin [19]. Hydrogenase enzyme catalyzes the reduction of protons to hydrogen, using electrons from ferredoxin. The two types of fermentation pathways are distinguished depending on which chemical compound is the main product. Bacillus and Enterobacter species have the ability to metabolize via mixed acid fermentation [20,21,22]. In this case, butyric acid, butanol, hydrogen, carbon dioxide, acetic acid and other compounds such as acetone may be formed. Generation of a variety of final products decreases the amount of formed hydrogen. Theoretically stoichiometric amount of hydrogen produced during the complete decomposition of 1 mol of glucose may be equal to 12 mol (Eq. 1).
Many factors such as inoculum, substrates, inorganic nutrients, reactor type and operational conditions like temperature and pH influence on the dark fermentation process course [23, 24]. Depending on the factors, different courses of fermentation may occur. When pure bacterial cultures are used, hydrogen is the main product during fermentation. However, using different types of inoculum, i.e., mixed sludge, causes a risk of infection even if the inoculum is prepared according to the procedures described in the literature [1, 2].
Considering all the analytical problems that are encountered in gas analysis during dark fermentation, there is a growing interest in analytical tools that can identify and determine the amount and composition of emerging gases online. Till now, gas analysis is usually based on sampling and laboratory analysis via gas chromatography (GC) [25, 26]. However, this approach may be time-consuming and expensive. A comparison of analytical- and sensor-based methods for gas analysis is given in Table 1.
In the monitoring of gaseous/odorogenic compounds, primarily olfactometry is used. This is a method of quantifying the aromatic concentration expressed in European fragrances in a cubic meter using an instrument called an olfactometer [27]. Gas chromatography is another technique used to analyze gas mixtures. It is used to separate and identify chemical compounds from a mixture. However, multi-sensor devices allow on a quick and in a easy way to detect and determine the intensifying odorogenic gases in a near real time as well as substances lacking a perceptible odor (i.e., carbon dioxide and water vapor) [28,29,30].
2 Experimental
2.1 Analytical methods
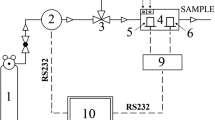
The composition and analysis of biogas were carried out using a gas chromatograph (PerkinElmer AutoSystem XL) with a Porapak Q column (100–120 mesh 6.5 m × 1/8, pressure 200 kPa) and an oven temperature of 60 °C. The studies were carried using a thermo-conductivity detector (TCD, temperature 100 °C). Nitrogen with a flow of 30 mL/min was used as the carrier gas. The volume of the analyzed sample was 0.2 mL. During the analysis (15 min), TurboChrom software was used.
2.2 Dark fermentation
Dark fermentation was carried out with the use of wastewater mixed sludge (municipal wastewater treatment plant, Saur Neptun Gdańsk, Poland) after Faloye procedure with respect to distilled glycerin as the sole carbon source. Faloye procedure allowed to destroy non-spherical microorganisms [1, 2]. Dark fermentation was carried in triplicate in sterile 1200 mL glass bioreactors with a working volume of 1000 mL fermentation broth and under regulated pH conditions as described in the previous study [26, 31, 32]. The initial fermentation broth was composed of 900 mL of 20.0 g/L solution of Buffered Peptone Water (Biomaxima Gdańsk, Poland) and 5.5 g/L distilled glycerin as a sole carbon source. Control (negative) was carried in 20.0 g/L solutions of Buffered Peptone Water (Biomaxima Gdańsk, Poland) without glycerin and glucose control with Thioglycollate (Biomaxima Gdańsk, Poland) with the addition of 5.5 g/L of glucose. In each flask, 100 mL of Faloye-pre-treated inoculum was introduced to the broth at 37 °C (Table 2). The pH of fermentation broth was adjusted to 7.00 with 1 M NaOH. Before inoculation, anaerobic conditions were created by purging the reactors with sterile nitrogen (Linde, purity 99.98%) for 60 min. Operational set-points were set at 37 °C and 320 RPM (rotations/min) for temperature and agitation, respectively [26]. The composition of fermentation broths is presented in Table 2.
3 Results and discussion
Anaerobic digestion is a multi-step process carried out by consortia of highly diversified microorganisms and requires strictly anaerobic conditions. Such conditions enable the transformation of organic matter into carbon dioxide and methane, if methanogenesis is inactive. In the first stage of AD, organic matter, i.e., glycerin, is hydrolyzed; then, in the second stage, it is converted via fermentative bacteria to a mixture of minor products. In the third stage, acetogenic bacteria convert minor products to acetate, CO2 and H2. Consequently, as the terminal phase of the culture occurs, methanogenesis takes place [33]. Different microbial populations have specific optimum working conditions and are inhibited by several process parameters such as pH, temperature, alkalinity, concentration of free ammonia, hydrogen, sodium, potassium, volatile fatty acids (abr.VFA) or heavy metals.
The changes in the composition of gas formed during dark fermentation on diversified feed material are presented in Fig. 2. During the experiment, all mentioned stages could be observed. When glucose was used as a sole carbon source (glucose control), no induction time concerning dark fermentation was observed. High concentrations of hydrogen were detected after 20 h of culture. The production of hydrogen lasted 96 h, after which the termination could be observed, as the hydrogen concentration dropped. In a similar broth, however, with a relatively lower glucose content (negative control), a small production of hydrogen was observed and methane was not detected. The course of dark fermentation for glycerin as a sole carbon source revealed an induction time. After 20 h of dark fermentation, the initiation phase passed to logarithmic growth phase. Hydrogen was generated till ca. 75 h of culture. In ca. 75 h of the culture, the hydrogen’s terminal phase occurred and methanogens started to produce methane. The concentrations of methane obtained from the culture were even higher than the ones obtained for hydrogen. The authors expected to detect other gases, i.e., H2S; however, its concentration was lower than the limits of detection in the used GC; therefore, a need to search for alternative gas composition determination has occurred. As the hydrogen productivity is concerned, it is crucial to determine the moment, when the hydrogen concentration drops and CH4 and H2S production occurs. The authors have placed a red line in the time point, for which the methane concentration starts to increase. From that moment, the produced gas stream needs to be separated, in order to keep a high purity of previously formed hydrogen. Unfortunately, the applied GC technique did not allow to determine the H2S concentration, as its level was under the limit of detection (abr. LOD). The same situation has taken place for other fermentation gases, i.e., ammonia, carbon monoxide and oxygen. In other studies, similar hydrogen productivity during dark fermentation was observed [34,35,36]. However, the data in the literature are very hard to compare, as they are normalized and often received in a diversified broths, volumes and with the application of varied process parameters, i.e., pH, temperature, agitation of even the type of wastewater sludge.
A delay in the GC analysis results may cause a delay in the hydrogen and methane streams separation. Therefore, the authors conducted that in order to carry out further tests, a review of commercially available gas sensors for the purposes of dark fermentation course analysis and gas composition determination is required.
On the basis of described research, the authors decided that further tests on the composition of fermentative gaseous products require alternative methods, i.e., gas sensors. Gas composition may be carried using a MOS type sensor, with CuO [37], which meets the criteria for typical fermentation gas measurements (Fig. 3). The sensor is selective for H2S concentration, but also has a relatively good response to other gases potentially contained in the tested mixture. Therefore, a research on sensors used for fermentation control needs to be carried. The criteria of selection should include the selectivity not only for the hydrogen, hydrogen sulfide or methane, but also the sensitivity to the response of other gases contained in the mixture—ammonia, carbon dioxide and oxygen.
Examples of commercially available gas sensors, that might be applicable for the gases obtained via dark fermentation, are presented in Table 2. Sensors that are commercially available can be obtained from companies such as Duran Electronica and Figaro. Tables 3 and 4 show the examples of gas sensors and chromatographic methods with and their technical specifications, respectively. After analyzing the concentration ranges in which the sensors operate, it can be confirmed that readily available gas sensors meet the requirements for monitoring the dark fermentation process. However, as conducted during the fermentation, gas chromatography allows us to control only gases such as hydrogen, carbon dioxide and methane.
Comparison of the possibilities of detection concerning interesting fermentation gaseous products defined for both gas sensors (Table 3) and gas chromatography (Table 4) reveals that it was not possible to detect H2S and NH3 using gas chromatography. However, it is possible to detect mentioned gases using sensor matrixes. The literature reports very low concentrations of H2S and NH3 in the gaseous products [43,44,45]. Therefore, the limits of detection on the level 0–300 ppm for H2S and 0–100 ppm for NH3 may be satisfactory for the control purposes of dark fermentation. After analyzing the concentration ranges in which the sensors operate, it can be confirmed that readily available gas sensors meet the requirements for monitoring the dark fermentation process. On the other hand, gas chromatography did not detect H2S or NH3, so it only allows to control gases such as hydrogen, carbon dioxide and methane. The presence of other mentioned products needs to be confirmed in further research.
4 Conclusions
Complexed matrix of gaseous components may be formed and revealed during the dark fermentation process. The authors confirmed the presence of hydrogen, carbon dioxide, methane and oxygen during the process. Hydrogen was mainly generated in the initial, lag and log phase of the culture and methane in the late logarithmic death phase of the culture, only when glycerin was used a sole carbon source. However, other fermentation gaseous products’ presence was not confirmed, as their concentration was under the limits of detection. The time point characterizing the moment when the methane production starts delivers some difficulties, as the gas chromatography delivers a slight delay regarding the results obtaining, as it is not a continuous method. As this time point is correlated with the appearance of CH4 and H2S, which occurs, when the metabolic pathway of bacteria and Archea take place, it seems crucial to determine other gases, not detectable using gas chromatography in the occurring levels. Therefore, a revision regarding the application of matrices sensors was proposed, and the levels of gases able to be measured using both gas chromatography and matrices sensors were conducted. The criteria of matrix sensors’ selection should include the selectivity not only for the hydrogen, hydrogen sulfide or methane, but also the sensitivity to the response of other gases contained in the mixture—ammonium, carbon dioxide and oxygen. A comprehensive combination of commercially available sensors and their applicability for the purposes of dark fermentation course analysis was presented on the basis of the levels of gas concentrations in the generated gas mixture. The authors conducted that the multi-sensor matrices may be a good alternative solution for fermentation gas measurement, especially when it is possible to apply them in a continuous matter. In addition, hydrogen sulfide and ammonia concentrations can be determined using gas sensors, which were under the limits of detection for gas chromatography. The application of sensor matrices for the dark fermentation course analysis needs to become a future step for the gas analysis and streams separation method development.
References
Faloye FD, Kana EBG, Schmidt S (2013) Optimization of hybrid inoculum development techniques for biohydrogen production and preliminary scale up. Int J Hydrog Energy 38:11765–11773. https://doi.org/10.1016/j.ijhydene.2013.06.129
Faloye FD, Kana EBG, Schmidt S (2014) Optimization of biohydrogen inoculum development via a hybrid pH and microwave treatment technique—semi pilot scale production assessment. Int J Hydrog Energy 39:5607–5616. https://doi.org/10.1016/j.ijhydene.2014.01.163
Ghimire A, Frunzo L, Pirozzi F, Trably E, Escudie R, Lens PNL et al (2015) A review on dark fermentative biohydrogen production from organic biomass: process parameters and use of by-products. Appl Energy 144:73–95. https://doi.org/10.1016/j.apenergy.2015.01.045
Sveinsdottir M, Beck SR, Orlygsson J (2009) Ethanol production from monosugars and lignocellulosic biomass by thermophilic bacteria isolated from Icelandic hot springs. Icel Agric Sci 22:45–58
Łukajtis R, Hołowacz I, Kucharska K, Glinka M, Rybarczyk P, Przyjazny A et al (2018) Hydrogen production from biomass using dark fermentation. Renew Sustain Energy Rev 91:665–694. https://doi.org/10.1016/J.RSER.2018.04.043
Kucharska K, Hołowacz I, Konopacka-Łyskawa D, Rybarczyk P, Kami M (2018) Key issues in modeling and optimization of lignocellulosic biomass fermentative conversion to gaseous biofuels. Renew Energy. https://doi.org/10.1016/j.renene.2018.06.018
Kucharska K, Rybarczyk P, Hołowacz I, Łukajtis R, Glinka M, Kamiński M (2018) Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 23:2937. https://doi.org/10.3390/molecules23112937
Kucharska K, Hołowacz I, Konopacka-Łyskawa D, Rybarczyk P, Kamiński M (2018) Key issues in modeling and optimization of lignocellulosic biomass fermentative conversion to gaseous biofuels. Renew Energy 129:384–408. https://doi.org/10.1016/j.renene.2018.06.018
Kabir MM, Rajendran K, Taherzadeh MJ, Horváth IS (2015) Experimental and economical evaluation of bioconversion of forest residues to biogas using organosolv pretreatment. Bioresour Technol 178:201–208. https://doi.org/10.1016/j.biortech.2014.07.064
Logan BE (2004) Extracting hydrogen and electricity from renewable resources. Environ Sci Technol 38:4–8
Sinha P, Pandey A (2011) An evaluative report and challenges for fermentative biohydrogen production. Int J Hydrog Energy 36:7460–7478. https://doi.org/10.1016/j.ijhydene.2011.03.077
Kapdan IK, Kargi F (2006) Bio-hydrogen production from waste materials. Enzyme Microb Technol 38:569–582. https://doi.org/10.1016/j.enzmictec.2005.09.015
Momirlan M, Veziroglu T (2002) Current status of hydrogen energy. Renew Sustain Energy Rev 6:141–179. https://doi.org/10.1016/S1364-0321(02)00004-7
Pääkkönen A, Tolvanen H, Rintala J (2018) Techno-economic analysis of a power to biogas system operated based on fluctuating electricity price. Renew Energy 117:166–174. https://doi.org/10.1016/j.renene.2017.10.031
Logan BE, Call D, Cheng S, Hamelers HVM, Sleutels THJA, Jeremiasse AW et al (2008) Microbial electrolysis cells for high yield hydrogen gas production from organic matter. Environ Sci Technol 42:8630–8640. https://doi.org/10.1021/es801553z
Nath K, Muthukumar M, Kumar A, Das D (2008) Kinetics of two-stage fermentation process for the production of hydrogen. Int J Hydrog Energy 33:1195–1203. https://doi.org/10.1016/j.ijhydene.2007.12.011
Oztekin R, Kapdan IK, Kargi F, Argun H (2008) Optimization of media composition for hydrogen gas production from hydrolyzed wheat starch by dark fermentation. Int J Hydrog Energy 33:4083–4090. https://doi.org/10.1016/j.ijhydene.2008.05.052
Ren NQ, Chua H, Chan SY, Tsang YF, Wang YJ, Sin N (2007) Assessing optimal fermentation type for bio-hydrogen production in continuous-flow acidogenic reactors. Bioresour Technol 98:1774–1780. https://doi.org/10.1016/j.biortech.2006.07.026
Uyeda K, Rabinowitz JC (1971) Pyruvate-ferredoxin oxidoreductase. IV. Studies on the reaction mechanism. J Biol Chem 246:3120–3125
Chong PS, Jahim JM, Harun S, Lim SS, Mutalib SA, Hassan O et al (2013) Enhancement of batch biohydrogen production from prehydrolysate of acid treated oil palm empty fruit bunch. Int J Hydrog Energy 38:9592–9599. https://doi.org/10.1016/j.ijhydene.2013.01.154
Yang P, Zhang R, McGarvey JA, Benemann JR (2007) Biohydrogen production from cheese processing wastewater by anaerobic fermentation using mixed microbial communities. Int J Hydrog Energy 32:4761–4771. https://doi.org/10.1016/j.ijhydene.2007.07.038
Mohan SV, Babu VL, Sarma PN (2008) Effect of various pretreatment methods on anaerobic mixed microflora to enhance biohydrogen production utilizing dairy wastewater as substrate. Bioresour Technol 99:59–67. https://doi.org/10.1016/j.biortech.2006.12.004
Favaro L, Alibardi L, Lavagnolo MC, Casella S, Basaglia M (2013) Effects of inoculum and indigenous microflora on hydrogen production from the organic fraction of municipal solid waste. Int J Hydrog Energy 38:11774–11779. https://doi.org/10.1016/j.ijhydene.2013.06.137
Hawkes FR, Hussy I, Kyazze G, Dinsdale R, Hawkes DL (2007) Continuous dark fermentative hydrogen production by mesophilic microflora: principles and progress. Int J Hydrog Energy 32:172–184. https://doi.org/10.1016/j.ijhydene.2006.08.014
Łukajtis R, Rybarczyk P, Kucharska K, Konopacka-Łyskawa D, Słupek E, Wychodnik K et al (2018) Optimization of saccharification conditions of lignocellulosic biomass under alkaline pre-treatment and enzymatic hydrolysis. Energies. https://doi.org/10.3390/en11040886
Łukajtis R, Kucharska K, Hołowacz I, Rybarczyk P, Wychodnik K, Słupek E et al (2018) Comparison and optimization of saccharification conditions of alkaline pre-treated triticale straw for acid and enzymatic hydrolysis followed by ethanol fermentation. Energies 11:639. https://doi.org/10.3390/en11030639
Grzelka A, Sówka I, Miller U (2018) Metody oceny emisji odorów z obiektów gospodarki hodowlanej. Inżynieria Ekologiczna 19:56–64
Sówka I (2011) Methods of identification of odour gases emitted from industrial plants. Prace Naukowe Instytutu Inżynierii Ochrony Środowiska Politechniki Wrocławskiej. Monografie vol 90, nr 55
Gebicki J, Dymerski T (2016) Application of chemical sensors and sensor matrixes to air quality evaluation, vol 73. Elsevier, New York. https://doi.org/10.1016/bs.coac.2016.02.007
Gebicki J (2016) Trends in analytical chemistry application of electrochemical sensors and sensor matrixes for measurement of odorous chemical compounds. Trends Anal Chem 77:1–13. https://doi.org/10.1016/j.trac.2015.10.005
Kucharska K, Łukajtis R, Słupek E, Cieśliński H, Rybarczyk P, Kamiński M (2018) Hydrogen production from energy poplar preceded by MEA pre-treatment and enzymatic hydrolysis. Molecules 23:1–21. https://doi.org/10.3390/molecules23113029
Łukajtis R, Hołowacz I, Kucharska K, Glinka M, Rybarczyk P, Przyjazny A et al (2018) Corrigendum to “Hydrogen production from biomass using dark fermentation” [Renew Sustain Energy Rev 91 (2018) 665–94]. Renew Sustain Energy Rev. https://doi.org/10.1016/j.rser.2018.06.030
Zheng Y, Zhao J, Xu F, Li Y (2014) Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog Energy Combust Sci 42:35–53. https://doi.org/10.1016/j.pecs.2014.01.001
Khaleb N, Jahim J, Kamal S (2012) Biohydrogen production using hydrolysates of palm oil mill effluent (POME). J Asian Sci 2:705–710
Zhang JN, Li YH, Zheng HQ, Fan YT, Hou HW (2015) Direct degradation of cellulosic biomass to bio-hydrogen from a newly isolated strain Clostridium sartagoforme FZ11. Bioresour Technol 192:60–67. https://doi.org/10.1016/j.biortech.2015.05.034
Zhu H, Béland M (2006) Evaluation of alternative methods of preparing hydrogen producing seeds from digested wastewater sludge. Int J Hydrog Energy 31:1980–1988. https://doi.org/10.1016/j.ijhydene.2006.01.019
Qin Y, Zhang F, Chen Y, Zhou Y, Li J, Zhu A et al (2012) Hierarchically porous CuO hollow spheres fabricated via a one-pot template-free method for high-performance gas sensors. J Phys Chem C 116:11994–12000. https://doi.org/10.1021/jp212029n
Bejaoui A, Guerin J, Zapien JA, Aguir K (2014) Theoretical and experimental study of the response of CuO gas sensor under ozone. Sens Actuators B Chem 190:8–15. https://doi.org/10.1016/J.SNB.2013.06.084
http://www.duranelectronica.com/docs/56_2069_I-fichasondeltox-v07.pdf. Accessed 17 Apr 2019
https://sgx.cdistore.com/. Accessed 17 Apr 2019
https://www.maritex.com.pl/product/attachment/33377/tgs2611.pdf. Accessed 17 Apr 2019
Isobe K, Koba K, Ueda S, Senoo K, Harayama S, Suwa Y (2011) A simple and rapid GC/MS method for the simultaneous determination of gaseous metabolites. J Microbiol Methods 84:46–51. https://doi.org/10.1016/j.mimet.2010.10.009
Song C, Liu Q, Ji N, Deng S, Zhao J, Kitamura Y (2017) Natural gas purification by heat pump assisted MEA absorption process. Appl Energy 204:353–361. https://doi.org/10.1016/j.apenergy.2017.07.052
Yang M, Zhang W, Rosentrater K (2017) Anhydrous ammonia pretreatment of corn stover and enzymatic hydrolysis of glucan from pretreated corn stover. Fermentation 3:9. https://doi.org/10.3390/fermentation3010009
Lin Z, Huang H, Zhang H, Zhang L, Yan L, Chen J (2010) Ball milling pretreatment of corn stover for enhancing the efficiency of enzymatic hydrolysis. Appl Biochem Biotechnol 162:1872–1880. https://doi.org/10.1007/s12010-010-8965-5
Funding
This work was carried out within the framework of the project “Studies of alkaline hydrolysis of lignocellulosic biomass and conversion conditions of hydrolyzed products to biogas”, supported financially by the National Science Center through the Grant UMO-2014/13/B/ST8/04258.
Author information
Authors and Affiliations
Contributions
ES and KK conceived, designed and carried the experiments. ES, KK, JG wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; and in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Słupek, E., Kucharska, K. & Gębicki, J. Alternative methods for dark fermentation course analysis. SN Appl. Sci. 1, 469 (2019). https://doi.org/10.1007/s42452-019-0488-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0488-2