Abstract
Diastasis recti abdominis (DRA) is a common occurrence in postpartum women, and it is unclear what types of nonsurgical interventions are most effective in preventing and/or reducing it. The aim of this review with meta-analysis was to investigate which conservative treatment approaches are the most effective for the management of postpartum DRA. After a thorough search of the PubMed and Scopus databases, we reviewed 14 articles. The literature suggests that abdominal exercise programs are generally effective in treating DRA at various postpartum periods. There is preliminary but promising evidence of the efficacy of electrical stimulation in combination with exercise. In addition, abdominal kinesiotaping can be used in conjunction with other interventions. Limitations of previous research include (a) the use of different measurement methods (palpation, calipers, ultrasound) and sites, (b) the evaluation of treatment effects in different time periods, and (c) the use of a wider range of exercise combinations. Although abdominal exercise is a cornerstone of DRA treatment, the optimal exercise combination is currently unknown due to these limitations.
Similar content being viewed by others
Introduction
During pregnancy, the two muscle bellies of the rectus abdominis muscle, which are connected by the linea alba, elongate and curve round as the abdominal wall expands to make room for the growing fetus [1, 2]. Diastasis of the rectus abdominis (DRA) occurs as a result of hormonal changes leading to elastic changes in the connective tissue, mechanical stresses on the abdominal wall from the growing fetus, and displacement of the abdominal organs [3, 4]. In most pregnant women, the inter-recti distance (IRD) increases due to stretching and thinning of the linea alba [5, 6]. This is a natural process and happens to some degree in most pregnancies. However, in some cases, the abdominal muscle changes may become excessive and persist after delivery [7]. DRA is defined as an excessive separation (i.e., increased IRD) between the bellies of the recti abdominis muscles. DRA can occur anywhere along the linea alba, from the xiphoid process to the public bone, and is quantified by the inter-recti distance [8, 9].
Studies have shown that IRD increases around the 14th week of gestation and continues to increase until delivery. Clinically significant DRA usually occurs early in the last trimester of pregnancy and its peak incidence is immediately after and in the first weeks following childbirth. The prevalence of DRA ranges from 32 to 95% in late pregnancy and 30–68% in the postpartum period [7]. After childbirth, the changes in the abdominal musculature, especially in the linea alba and rectus abdominis sheath, usually return to normal and have no significant impact on health [10]. Recovery is most marked between 1 day and 8 weeks after delivery, followed by a plateau phase, and if no interventions are performed, IRD may remain elevated throughout life [11].
The abdominal wall plays an important role in posture and postural control, trunk and pelvic stability, respiration, trunk movement, and support of the abdominal viscera [1]. Persistent DRA can potentially lead to a range of symptoms and dysfunctions [10, 12]. These can manifest as altered trunk mechanics, impaired pelvic stability, and altered posture, which can make the lumbar spine and pelvis more susceptible to injury. Previous research has also found that DRA is related to low back pain [13], possibly due to associated decrements in trunk muscle strength, lumbopelvic instability, and pelvic floor weakness [14, 15]. A variety of factors have been shown to increase the risk of DRA, including multiparity, maternal age, body mass index before pregnancy, and at 6 months postpartum, weight gain during pregnancy, Beighton’s hypermobility score, baby weight at birth, abdominal circumference at gestational week 35, exercise training level before, during and after pregnancy, and type of delivery [16].
Women with DRA are usually referred to physical therapists for conservative treatment [17]. However, little is known about the optimal training methods for treating DRA. A 2014 review acknowledged that prenatal exercise can reduce the incidence of DRA by 35% [18]. Anecdotally, regular exercise before pregnancy and during the prenatal period appears to decrease the risk of developing DRA and reduce IRD [18, 19]. Abdominal exercises targeting the trunk flexors are commonly prescribed for women with postpartum DRA. Other regularly used nonsurgical interventions include postural and back training, external support, and aerobic exercise [20,21,22]. However, it remains unclear which types of nonsurgical interventions are most effective in preventing and/or reducing DRA. Therefore, the aim of this review was to determine which conservative treatment approaches may be most effective in reducing IRD in postpartum women.
Methods
Inclusion Criteria
Study inclusion criteria were structured according to PICOS tool [23]:
-
Population (P): postpartum females
-
Intervention (I): exercise or other non-pharmacological interventions intended for treating DRA
-
Comparisons (C): control groups, receiving no intervention or placebo intervention; or comparisons of two different interventions
-
Outcomes (O): Inter-rectus distance
-
Study design (S): randomized controlled trials and non-randomized clinical trials
Search Strategy
PubMed and Scopus databases were searched in January 2022 without regard to the date of publication. We used the following combination of search key words: (diastasis recti OR stress urinary incontinence OR abdominal separation OR urinary incontinence) AND (exercise OR therapy OR intervention) AND (pregnancy OR pregnant OR postpartum) AND (months OR weeks OR follow-up). Additionally, reference lists of several review articles describing interventions for older adults were carefully scrutinized. Finally, we performed a backward and forward citation search; specifically, we reviewed reference lists of all articles that were already retrieved through the database, and we also reviewed all papers that have cited them, using the Google Scholar platform. Potentially relevant articles were screened in full text, followed by additional screening for their eligibility by the additional reviewers.
Data Extraction
The extracted data included (a) baseline and post-intervention means and standard deviations for IRD; (b) baseline demographics of participants (gender, age, body height, body mass, body mass index); (c) intervention characteristics, such as duration of the intervention, number of sessions per week, volume (number of exercises, sets, and repetitions), breaks between exercises and sets, supervision, and progression of exercise difficulty); (d) IRD measurement characteristics (method, inclusion of contraction, measurement location, number of trials). Regarding the latter, previous studies have differed in regard to IRD measurement method (ultrasound, caliper, manual measurements), location (4.5 cm above and below the umbilicus being the most common) and inclusion of contraction (either measuring IRD during abdominal contraction or in relaxed condition). Data were carefully entered into Microsoft Excel 2016 (Microsoft, Redmond, WA, USA). If the data were presented in a graphical rather than tabular form, we used Adobe Illustrator Software (version CS5, Adobe Inc., San Jose, CA, USA) to accurately determine the means and standard deviations. In case of missing data, the corresponding author of the respective articles was contacted by e-mail. If no response was received after 21 days, the author was contacted again. If the author did not reply to the second inquiry, the data was considered irretrievable.
Data Analysis and Synthesis
The main data analyses were carried out in Review Manager (Version 5.3, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014, London, UK). Before the results were entered into the meta-analytical model, the pre-post differences and pooled standard deviations were calculated according to the following formula SD = √[(SDpre2 + SDpost2) − (2 × r × SDpre × SDpost). The correction value (r), which represents the pre-test–post-test correlation of outcome measures, was conservatively set at 0.75. It should be noted that a change in the correction value in the range between 0.5 and 0.9 had little effect on the pooled SD and would not change the outcomes of the meta-analyses. For the meta-analyses, the inverse variance method for continuous outcomes with a random-effects model was used. The effect sizes were expressed as standardized mean difference (SMD). The respective 95% confidence intervals were also calculated and reported. Even when only one study was considered, we calculated the SMD to enable the comparison of effect sizes across studies. We conducted separate analyses depending on the location of measurement and contraction (i.e., measurement with and without muscle contraction), but not the measurement method. Statistical heterogeneity among studies was determined by calculating the I2 statistics. According to Cochrane guidelines, the I2 statistics of 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity, and 75% to 100% indicates considerable heterogeneity [24]. The threshold for statistical significance was set at p ≤ 0.05 for the main effect size and the subgroup difference tests.
Results
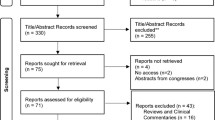
In total, 975 records from the PubMed database and 423 from the Scopus database were retrieved (total n = 1398). After the removal of the duplicates, 1112 records remained for screening. Based on the examination of the titles, 215 papers were left for the examination of the abstract, after which 22 papers were chosen for full-text evaluation. After reading the full texts, 13 papers were included in the systematic review. One additional paper was identified in the reference list of previous papers, for a total of 14 papers reviewed. The procedure is shown in Fig. 1 in detail. The overview of the included studies is provided in Table 1.
The Effect of Exercise on IRD At Rest
Only two studies with exercise-based interventions and control groups (58 participants in total) assessed IRD above the umbilicus (4.5 cm in both) at rest [25, 26]. The pooled SMD was 2.39, but was not statistically significant (CI: − 0.67, 5.42; p = 0.13; I2 = 92%). However, one of the studies [26] was done on 8 participants only, which included women at 6–24 months postpartum. The other study (n = 25 per group; 3–6 months postpartum) showed a large effect of plank exercise (SMD = 3.91, CI = 2.93–4.88, p < 0.011). The same two studies also assessed IRD 4.5 cm below the umbilicus at rest. The pooled effect was again not statistically significant (SMD = 1.27; CI: − 1.34 to 3.88; p = 0.340; I2 = 91%). Again, the first study [25] showed a significant reduction in IRD in the plank exercise group (SMD = 2.54, CI: 1.78–3.30, p < 0.001).
Effect of Exercise on IRD During Contraction
Three studies (98 participants in total) assessed IRD above the umbilicus during voluntary contraction [25,26,27]. The meta-analysis indicated large reductions of IRD in the experimental groups (SMD = 2.85; CI: 0.74–4.97; p = 0.008; I2 = 90%). The removal of the small study with 8 participants only [26] resulted in even larger and more consistent effect (SMD = 3.89; CI = 1.92–5.86; p < 0.001; I2 = 85%). Two studies [25, 26] also assessed IRD below the umbilicus during rectus abdominis contraction, and showed a completely opposite effects (SMD = 2.31 and − 1.16, respectively), making the pooled effect small and non-significant (SMD = 0.65; CI: -2.74 to 4.14). The former study [25] with a larger sample size showed a very large beneficial effect of exercise (SMD = 2.31; CI: 1.58–3.03; p < 0.001).
One study [28] without a control group investigated the effect of hypopressive exercise on IRD in 12 women at 2 or more months postpartum. The authors reported statistically significant reductions in IRD (p < 0.05). Assuming a null effect in a potential control group, the effect would translate into large SMD (3.22; CI: 1.94–4.50). An additional study (including 15 postpartum women) with exercise-based intervention (abdominal exercise) and no control group was conducted [29]. Assuming a null effect in a potential control group, the effect would translate into large SMD for the measurement above (2.04; CI: 1.02–3.05) and below the umbilicus (2.09; CI: 1.06–3.12) during contraction.
One study did not measure the exact IRD, but reported only the prevalence of DRA [30]. The participants (n = 157; women 6 weeks postpartum) were randomized in experimental and control group. The participants in the experimental group received supervised pelvic floor exercises and were encouraged to also perform the program at home, while the control group only received 1 session with exercise instructions. The prevalence of DRA was reduced similarly in both groups (from 54–55 to 30–41%), indicating no differences between the groups.
Effect of Exercise Interventions with Undefined/Unclear IRD Measurement
Two studies [31, 32] assessed the effect of exercise interventions (56 participants in total, women 0–3 weeks postpartum), but did not specify the details regarding their IRD assessments. The pooled effect was not statistically significant (SMD = 0.94; CI: − 1.64, 3.94; p = 0.5), as the studies showed the opposite effect. In the first study [31], exercise induced reductions in IRD statistically significantly larger than in the passive control group (SMD = 2.32; CI: 1.50–3.15; p < 0.001), however, there was no difference between exercise and control groups in the second study [32] (SMD = − 0.47; − 1.47 to 0.53; p = 0.360). In addition, abdominal crunch exercise was very effective to improve IRD in postpartum women (SMD = 4.47; CI: 3.26–5.57) in another study [33], who used caliper measurements, but did not specify if the IRD was assessed at rest or during contraction.
Other Interventions
One study [34] investigated the immediate effect of kinesiotaping on IRD in 12 women 6–12 weeks postpartum. The IRD reduction was statistically significant for the measurement above the umbilicus (SMD = 1.12; CI = 0.25–2.00; p = 0.01), but not for the measurement below the umbilicus (SMD = 0.62; CI = − 0.20–1.45; p = 0.140).
One study compared neuromuscular electrical stimulation and abdominal exercise, to abdominal exercise alone, with 30 participants (women at least 2 months postpartum) per group [35]. The parameters for electrical stimulation were: a frequency 80 pulses/min, with pulse width 0.1–0.5 ms, and as on:off ratio of 5 s:10 s for the total stimulation time of 30 min. The analysis showed that the combination of neuromuscular electrical stimulation and abdominal exercise decreased IRD statistically significantly more than exercise alone (SMD = 2.88; CI: 2.14–3.61; p < 0.001). Another study assessed the effect of electrical stimulation followed by exercise in comparison to no intervention [36]. The IRD decreased in the intervention group only (from 3.1 ± 0.7 cm to 2.4 ± 0.7 above the umbilicus, and 2.0 ± 0.6 cm to 1.7 ± 0.6 cm below the umbilicus) [36].
Two studies (46 participants in total; 0–3 weeks postpartum) compared wearing abdominal belt or abdominal binding with exercise-based interventions [32, 37]. The first study strongly favored the exercise interventions (SMD = 2.51; CI: 1.52–3.50; p < 0.001), while there were no statistically significant differences between groups in the second study (SMD = − 0.41; CI: − 1.49 to 0.51; p = 0.340), although the direction of the effect favored the use of abdominal binding. The pooled effect was not statistically significant (SMD = 1.01; CI: − 1.93 to 3.95; p = 0.501; I2 = 94%). In the latter study, the effect of combined exercise and abdominal binding was not superior to abdominal binding alone (SMD = 0.12; p = 0.822).
One study compared the effectiveness of online (e-platform based) and offline core stabilization exercise protocols for reducing IRD. During the 6 weeks of intervention, the two groups performed the program twice for 40 min. For the online group, the exercise sessions were conducted through Zoom. In both groups, it was recommended that the exercise programs are implemented as often as possible. The IRD decreased from 1.99 ± 0.26 cm to 1.37 ± 0.40 cm (p < 0.001) in the online group and from 1.92 ± 0.30 cm to 1.18 ± 0.30 cm in the offline group (p < 0.001), with no differences observed between the groups (p > 0.05) [38].
Discussion
The aim of this review was to determine the scope of existing interventions for DRA in postpartum women and to compare their effectiveness in reducing IRD. The literature indicates that DRA is very common in pregnant and postpartum women. Yet, there are no clear and effective exercise-based approaches that can be recommended to women with DRA. With this in mind, we decided to conduct a review of previously studied interventions. We found only a handful of intervention studies, with different approaches and different measurement methods, making it difficult to compare results. Data from the studies included in this paper suggest that abdominal exercise plays a central role in reducing IRD and associated symptoms. Preliminary evidence suggests the potential of electrical stimulation as a complementary tool in the rehabilitation of IRD. In addition, online-based exercise programs appear to be as effective as on-site programs, which is important in light of potential limitations of group exercise programs, such as during the COVID -19 pandemic.
The major drawback of the current literature is the discrepancy in IRD measurement methods, as we found studies in which DRA was measured at rest or during contraction, as well as studies with undefined protocols. Some studies lacked control groups [28] and one study reported only the prevalence of DRA [30]. In addition to exercise-based interventions, one study examined the efficacy of kinesiotaping [34] and two studies examined the effect of neuromuscular electrical stimulation in combination with exercise [35, 36]. Two studies compared abdominal binding with exercise-based intervention [32, 37]. Because these studies had very different designs and interventions, quantitative statistical analysis within study groups (IRD at rest/IRD during contraction/undefined IRD measurement/other interventions) was limited.
In the group of studies that examined IRD at rest, no statistically significant pooled effects were observed for IRD either above or below the umbilicus. However, a large reduction in IRD was achieved in one study that used progressive prone plank exercise [25]. The group of interventions in which IRD was measured during voluntary contraction showed a large reduction in IRD above the umbilicus but not below the umbilicus. Because of these discrepancies, the pooled effect was small and not significant. Intervention studies that used other interventions, methods such as kinesiotaping, neuromuscular electrical stimulation, and/or abdominal exercise and wearing an abdominal belt with/without exercise found different effects. Kinesiotaping showed improvement above the umbilicus but not bellow [34], neuromuscular electrical stimulation was effective in combination with abdominal exercise [35] and the abdominal binding showed inconsistent effects when combined with exercise [32, 37].
Regardless of whether IRD was measured at rest or during contraction, the studies showed a decrease in IRD in several intervention groups. Therapeutic exercises are designed to activate both fast twitch and slow twitch fibers of the skeletal muscles, and they affect the metabolic demand needed to produce a given muscle force, which leads to increase in muscular power and endurance [39]. Recently, it has been shown that individuals with increased IRD have lower muscle stiffness in the rectus abdominis and transversus abdominis muscles, which is another indication of the importance of strengthening the abdominal muscles [40]. Some authors suggested that an increased mind-muscle connection (i.e., meaningful muscle activation) may contribute to the observed improvement [26]. Studies indicated the importance of activating the transversus abdominis muscle, as it brings together the bellies of the rectus abdominus muscle, improves the integrity of the linea alba, and increases fascial tension, thereby reducing DRA [41]. Interestingly, several studies showed that the changes in IRD were not uniform along the linea alba. The improvements limited to a specific region along the linea alba demonstrate the potential for rehabilitation, but also require further research to optimize exercise interventions.
Ideally, carefully designed rehabilitation exercises have the potential to positively impact DRA and related outcomes in the postpartum period, but this area appears to be under-researched. The studies we included in this meta-analysis also had a number of shortcomings that make conclusions difficult. This may be due, in part, to the different methods used to measure IRD. The studies we examined used different points along the linea alba for IRD measurement. Caution should be used when interpreting results measured at different points, because IRD magnitudes can vary considerably. A recent systematic review showed that the average IRD distance of the nullipara was 8.77 mm, the distance in the epigastric region was 7.22 mm, and that in the infraumbilical region was 4.09 mm [42]. Although IRD measurements are generally reliable and valid [43], the methods used to assess IRD were different in the various studies and were performed either at rest or during contraction (or nonspecifically), which may have affected the validity of our comparisons. Some, but not all, of these variables could be accounted for in the meta-analytic models. Some research also suggests that measuring the integrity (i.e., depth) of the linea alba in conjunction with IRD may be a more comprehensive approach to determining the severity of pregnancy-related DRA [44]. In recent years, ultrasonography-based measurements are gaining recognition as a reliable tool for measuring IRD [45]. Small and very different sample sizes in the studies is one of the major limitations despite good study designs. In addition, the comparison between the studies is confounded by the natural recovery, which is particularly evident in early postpartum period [7].
Conclusions
Given the prevalence of DRA, more research is needed on potential effective interventions. From existing publications, abdominal exercises are at the forefront of effective protocols to reduce IRD. Future interventions should consider the use of more uniform IRD measurement devices, preferably using ultrasound or calipers, which are more reliable than manual methods. Because of the impact that DRA can have on postpartum women’s quality of life, strategies to assess the severity of DRA and the integrity of the linea alba should be incorporated into postpartum health care. This would allow timely determination of whether DRA is present so that women can receive appropriate referral/treatment.
References
Gilleard WL, Brown JMM. Structure and function of the abdominal muscles in primigravid subjects during pregnancy and the immediate postbirth period. Phys Ther. 1996;76:750–62.
Axer H, Keyserlingk DGV, Prescher A. Collagen fibers in linea alba and rectus sheaths: I. General scheme and morphological aspects. J Surg Res. 2001;96:127–34.
Blaschak MJ, Boissonnault JS. Incidence of diastasis recti abdominis during the childbearing year. Phys Ther. 1988;68:1082–7.
Rath AM, Attali P, Dumas JL, Goldlust D, Zhang J, Chevrel JP. The abdominal linea alba: an anatomo-radiologic and biomechanical study. Surg Radiol Anat. 1996;18:281–8.
Beer GM, Schuster A, Seifert B, Manestar M, Mihic-Probst D, Weber SA. The normal width of the linea alba in nulliparous women. Clin Anat. 2009;22:706–11.
Barbosa S, De Sá RAM, Coca Velarde LG. Diastasis of rectus abdominis in the immediate puerperium: correlation between imaging diagnosis and clinical examination. Arch Gynecol Obstet. 2013;288:299–303.
Rett M, Braga M, Bernardes N, Andrade S. Prevalence of diastasis of the rectus abdominis muscles immediately postpartum : comparison between primiparae and multiparae. Rev Bras Fisioter. 2009;13:275–80.
da Mota PGF, Pascoal AGBA, Carita AIAD, Bø K. Prevalence and risk factors of diastasis recti abdominis from late pregnancy to 6 months postpartum, and relationship with lumbo-pelvic pain. Man Ther. 2015;20:200–5.
Sperstad JB, Tennfjord MK, Hilde G, Ellström-Engh M, Bø K. Diastasis recti abdominis during pregnancy and 12 months after childbirth: prevalence, risk factors and report of lumbopelvic pain. Br J Sports Med. 2016;50:1092–6.
Hills NF, Graham RB, McLean L. Comparison of trunk muscle function between women with and without diastasis recti abdominis at 1 year postpartum. Phys Ther. 2018;98:891–901.
Liaw L-J, Hsu M-J, Liao C-F, Liu M-F, Hsu A-T. The relationships between inter-recti distance measured by ultrasound imaging and abdominal muscle function in postpartum women: a 6-month follow-up study. J Orthop Sports Phys Ther. 2011;41:435–43.
Zappile-Lucis M. Quality of life measurements and physical therapy management of a female diagnosed with diastasis recti abdominis. J Womens Health Phys Therap. 2009;33:22.
Puri J, Sharma S, Samuel AJ, Chahal A. Investigate correlation between diastasis of rectus abdominis muscle and low back pain in obese women. J Lifestyle Med. 2021;11:38–42.
Yuan S, Wang H, Zhou J. Prevalence and risk factors of low back and pelvic pain in women with rectus abdominis diastasis: a multicenter retrospective cohort study. Korean J Pain. 2022;35:86–96.
Gluppe S, Ellström Engh M, Kari B. Women with diastasis recti abdominis might have weaker abdominal muscles and more abdominal pain, but no higher prevalence of pelvic floor disorders, low back and pelvic girdle pain than women without diastasis recti abdominis. Physiother (United Kingdom). 2021;111:57–65.
Cavalli M, Aiolfi A, Bruni PG, Manfredini L, Lombardo F, Bonfanti MT, et al. Prevalence and risk factors for diastasis recti abdominis: a review and proposal of a new anatomical variation. Hernia. 2021;25:883–90.
Keeler J, Albrecht M, Eberhardt L, Horn L, Donnelly C, Lowe D. Diastasis recti abdominis: a survey of women’s health specialists for current physical therapy clinical practice for postpartum women. J Women’s Heal. 2012;36:131–42.
Benjamin DR, van de Water ATM, Peiris CL. Effects of exercise on diastasis of the rectus abdominis muscle in the antenatal and postnatal periods: a systematic review. Physiother (United Kingdom). 2014;100:1–8.
Chiarello CM, Falzone LA, McCaslin KE, Patel MN, Ulery KR. The effects of an exercise program on diastasis recti abdominis in pregnant women. J Womens Health Phys Therap. 2005;29:11–6.
Sheppard S. Part I: management of postpartum gross divarication recti. J Assoc Chart Physiother Womens Heal. 1996;79:22–4.
Thornton SL, Thornton SJ. Management of gross divarication of the recti abdominis in pregnancy and labour. Physiother (United Kingdom). 1993;79:457–8.
Pascoal AG, Dionisio S, Cordeiro F, Mota P. Inter-rectus distance in postpartum women can be reduced by isometric contraction of the abdominal muscles: a preliminary case-control study. Physiother (United Kingdom). 2014;100:344–8.
Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014;14:579.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. Cochrane Handb Syst Rev Interv. John Wiley & Sons, 2019.
Awad E, Mobark A, Zidan AA, Hamada HA, Shousha T. Effect of progressive prone plank exercise program on diastasis of rectus abdominis muscle in postpartum women: a randomized controlled trial. J Hum Sport Exerc. 2021;16:395–403.
Laframboise FC, Schlaff RA, Baruth M. Postpartum exercise intervention targeting diastasis recti abdominis. Int J Exerc Sci. 2021;14:400–9.
Thabet AA, Alshehri MA. Efficacy of deep core stability exercise program in postpartum women with diastasis recti abdominis: a randomised controlled trial. J Musculoskelet Neuronal Interact. 2019;19:62–8.
Ramírez-Jiménez M, Alburquerque-Sendín F, Garrido-Castro JL, Rodrigues-de-Souza D. Effects of hypopressive exercises on postpartum abdominal diastasis, trunk circumference, and mechanical properties of abdominopelvic tissues: a case series. Physiother Theory Pract. 2021;Ahead of print.
Vaishnavi G, Mohan Kumar G, Jayson CJ, Kirupa K, Tharani G, Kamatchi K, et al. Effectiveness of exercise in treating rectus abdominis diastasis. Biomed. 2018;38:583–7.
Gluppe SL, Hilde G, Tennfjord MK, Engh ME, Bø K. Effect of a postpartum training program on the prevalence of diastasis recti abdominis in postpartum primiparous women: a randomized controlled trial. Phys Ther. 2018;98:260–8.
Bobowik PŻ, Dąbek A. Physiotherapy in women with diastasis of the rectus abdominis muscles. Adv Rehabil. 2018;32:11–7.
Keshwani N, Mathur S, McLean L. The impact of exercise therapy and abdominal binding in the management of diastasis recti abdominis in the early postpartum period: a pilot randomized controlled trial. Physiother Theory Pract. 2021;37:1018–33.
Saleem Z, Khan AA, Farooqui SI, Yasmeen R, Rizvi J. Effect of exercise on inter-recti distance and associated low back pain among postpartum females; a randomized controlled trial. J Fam Reprod Heal. 2021;15:202–9.
Ptaszkowska L, Gorecka J, Paprocka‐borowicz M, Walewicz K, Jarzab S, Majewska‐pulsakowska M, et al. Immediate effects of kinesio taping on rectus abdominis diastasis in postpartum women—preliminary report. J Clin Med. 2021;10:5043.
Kamel DM, Yousif AM. Neuromuscular electrical stimulation and strength recovery of postnatal diastasis recti abdominis muscles. Ann Rehabil Med. 2017;41:465–74.
Wei R, Yu F, Ju H, Jiang Q. Effect of electrical stimulation followed by exercises in postnatal diastasis recti abdominis via MMP2 gene expression. Cell Mol Biol. 2021;67:82–8.
El-Mekawy H, Eldeeb A, El-Lythy M, El-Begawy A. Effect of abdominal exercises versus abdominal supporting belt on postpartum abdominal efficiency and rectus separation. Int Sci Index [Internet]. 2013;7:44–8.
Kim S, Yi D, Yim J. The effect of core exercise using online videoconferencing platform and offline-based intervention in postpartum woman with diastasis recti abdominis. Int J Environ Res Public Health. 2022;19:7031.
Reiman MP, Lorenz DS. Integration of strength and conditioning principles into a rehabilitation program. Int J Sports Phys Ther [Internet]. 2011;6:241–53.
He K, Zhou X, Zhu Y, Wang B, Fu X, Yao Q, et al. Muscle elasticity is different in individuals with diastasis recti abdominis than healthy volunteers. Insights Imaging. 2021;12:1–11.
Mota P, Pascoal AG, Carita AI, Bø K. The immediate effects on inter-rectus distance of abdominal crunch and drawing-in exercises during pregnancy and the postpartum period. J Orthop Sports Phys Ther United States. 2015;45:781–8.
Wang Y, Wang H. Systematic review and meta-analysis of the inter-recti distance on ultrasound measurement in nulliparas. J Plast Surg Hand Surg. 2022.
Benjamin DR, Frawley HC, Shields N, Georgiou C, Taylor NF. Establishing measurement properties in the assessment of inter-recti distance of the abdominal muscles in a postnatal women. Musculoskelet Sci Pract. 2020;49:102202.
Dufour S, Hurd A, Lis E, Speckley J, Stotesbury A, Wright C. Pregnancy-related diastasis rectus abdominis: impact of a multi-component group-based intervention. Obstet Gynecol Int J. 2019;10:87–93.
Qu E, Wu J, Zhang M, Wu L, Zhang T, Xu J, et al. The ultrasound diagnostic criteria for diastasis recti and its correlation with pelvic floor dysfunction in early postpartum women. Quant Imaging Med Surg. 2021;11:706–13.
Author information
Authors and Affiliations
Contributions
IW and NS conceptualized the paper. IW and ZK performed the literature review. ZK performed the data analysis. IW and NS wrote the first manuscript draft. All authors worked on finalizing the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Medicine
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Weingerl, I., Kozinc, Ž. & Šarabon, N. The Effects of Conservative Interventions for treating Diastasis Recti Abdominis in Postpartum Women: a Review with Meta-analysis. SN Compr. Clin. Med. 5, 10 (2023). https://doi.org/10.1007/s42399-022-01353-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s42399-022-01353-4