Abstract
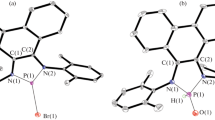
In this work, we describe the synthesis of O,P-heterocycles with sizes of 5, 6 and 8 atoms. A one pot-two steps processes accounts for the preparation of 1,2-oxaphosphinane and 1,2-oxaphosphacane. The synthesis of 1,2-oxaphospholane requires two steps involving the installation of functional group on the phosphinate introducing a P-O bond followed by the formation of a Pd-catalyzed C-P bond formation. Their molecular structures were assessed by single crystal X-ray diffraction. The geometries of the studied compounds were optimized using density functional theory (DFT) at the B3LYP and M06-2X hybrid functionals and compared to the experimental solid state data. In addition, Hirchfeld surfaces and frontier molecular orbitals were described.












Similar content being viewed by others
References
Binyamin I, Meidan-Shani S, Ashkenazi Beilstein N (2015) Synthesis of γ-hydroxypropyl P-chirogenic (±)-phosphorus oxide derivatives by regioselective ring-opening of oxaphospholane 2-oxide precursors. J Org Chem 11:1332–1339. https://doi.org/10.3762/bjoc.11.143
Kiss NZ, Keglevich G (2019) Direct esterification of phosphinic and phosphonic acids enhanced by ionic liquid additives. Pure Appl Chem 91:59–65. https://doi.org/10.1515/pac-2018-1008
Berger O, Montchamp JL (2014) Phosphinate-containing heterocycles: a mini-review. Beilstein J Org Chem 10:732–740. https://doi.org/10.3762/bjoc.10.67
Chaudhry A, Harger MGP, Shuff P, Thompson A (1999) Intramolecular nucleophilic displacement of halogen by phosphinate and thiophosphinate anions: relative rates of formation of five- and six-membered rings. J Chem Soc Perkin Trans 1:1347–1352. https://doi.org/10.1039/A900623K
Renard PY, Vayron P, Mioskowski C (2003) Toward antibody-catalyzed hydrolysis of organophosphorus poisons. Org Lett 5:1661–1664. https://doi.org/10.1073/pnas.97.13.7058
Xu J (2023) Synthesis of 1,2-oxaphosphinane 2-oxides and 1,2-oxaphosphinine 2-oxides: δ-phosphonolactones and δ-phosphinolactones. New J Chem 47:5441–5469. https://doi.org/10.1039/d2nj06106f
Qiao MM, Liu YY, Yao S, Ma TC, Tang ZL, Shi DQ, Xiao WJ (2019) A photoredox/cobalt-catalyzed phosphinyloxy radical addition/cyclization cascade: synthesis of phosphaisocoumarins. J Org Chem 11:6798–6806. https://doi.org/10.1021/acs.joc.9b00570
Eom D, Jeong Y, Kim YR, Lee E, Choi W, Lee PH (2013) Palladium-catalyzed C(sp2 and sp3)–H activation/C–O bond formation: synthesis of benzoxaphosphole 1- and 2-oxides. Org Lett 15:5210–5213. https://doi.org/10.1021/ol402736v
Shin S, Jeong Y, Jeon WH, Lee PH (2014) Phosphaannulation by palladium-catalyzed carbonylation of C–H bonds of phosphonic and phosphinic acids. Org Lett 16:2930–2933. https://doi.org/10.1021/ol501066k
Rodriguez VY, Del Aguila MA, Iglesias MJ, Ortiz FL (2012) Directed ortho-lithiation of unprotected diphenylphosphinic acids. Tetrahedron 68:7355–7362. https://doi.org/10.1016/j.tet.2012.06.088
Fu Z, Fu X, Du C, Xu J (2020) Microwave-assisted periselective annulation of triarylphosphenes with aldehydes and ketones. Org Biomol Chem 18:9526–9537. https://doi.org/10.1039/D0OB02011G
Hong F, Xia J, Xu JJ (1994) Palladium-catalysed carbocyclization of organophosphorus compounds: a novel and effective method for the synthesis of cyclic organophosphorus compounds including the phosphorus analogues of α-methylene lactones. Chem Soc Perkin Trans 1:1665–1666. https://doi.org/10.1039/P19940001665
Majumbar KC, Nandi RK, Ganai S (2014) Synthesis of phosphorus containing medium ring heterocycles by sequential Claisen rearrangement and ring closing metathesis. Tetrahedron Lett 55:1247–1250. https://doi.org/10.1016/j.tetlet.2014.01.008
Keglevich G, Jablonkai E, Balázs BL (2014) A “green” variation of the Hirao reaction: the P-C coupling of diethyl phosphite, alkyl phenyl-H-phosphinates and secondary phosphine oxides with bromoarenes using a P-ligand-free Pd(OAc)2 catalyst under microwave and solvent-free conditions. RSC Adv 4:22808–22816. https://doi.org/10.1039/C4RA03292F
Montchamp JL, Dumond R (2001) Synthesis of monosubstituted phosphinic acids: palladium-catalyzed cross-coupling reactions of anilinium hypophosphite. J Am Soc 123:510–511. https://doi.org/10.1021/ja005721c
Kalek M, Stawinski J (2009) Efficient synthesis of mono- and diarylphosphinic acids: a microwave-assisted palladium-catalyzed cross-coupling of aryl halides with phosphinate. Tetrahedron 50:10406–10412. https://doi.org/10.1016/j.tet.2009.10.028
Chen YR, Duan WL (2014) Palladium-catalyzed intramolecular direct arylation for phosphorus heterocycle synthesis. Synthesis 46:1067–1072. https://doi.org/10.1055/s-0033-1340832
Miles JA, Grabiak RC, Cummina C (1982) Synthesis of novel phosphorus heterocycles: 1,3-dihydro-2,1-benzoxaphosphole 1-oxides. J Org Chem 47:1677–1682. https://doi.org/10.1021/jo00348a013
Gholivand K, Védova COD, Erben MF, Mahzouni HR, Amiri ZSS (2008) Synthesis, spectroscopic study, X-ray crystallography and ab initio calculations of the two new phosphoramidates: C6H5OP(O)(NHC6H11)2 and [N(CH3)(C6H11)]P(O)(2–C5H4N-NH)2. J Mol Struct 874:178–186. https://doi.org/10.1016/j.molstruc.2007.03.047
Jones RO, Gunnarsson O (1989) The density functional formalism, its applications and prospects. Rev Mod Phys 6:689–746. https://doi.org/10.1103/RevModPhys.61.689
Liu Y, Zhao J, Li F, Chen Z (2013) Appropriate description of intermolecular interactions in the methane hydrates: an assessment of DFT methods. J Comput Chem 34:121–131. https://doi.org/10.1002/jcc.23112
Gaussian 09, Revision A.1, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian, Inc., Wallingford CT
Stephens PJ, Devlin JF, Chabalowski CF, Frish MJ (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98:11623–11627. https://doi.org/10.1021/j100096a001
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241. https://doi.org/10.1007/s00214-007-0310-x
Andersson MPP (2005) Uvdal new scale factors for harmonic vibrational frequencies using the B3LYP density functional method with the triple-ζ basis set 6–311+G(d, p). J Phys Chem A 109:12. https://doi.org/10.1021/jp045733a
Spackman MA, Byrom PG (1997) A novel definition of a molecule in a crystal. Chem Phys Lett 267:215–220. https://doi.org/10.1016/S0009-2614(97)00100-0
Chavda BR, Socha BN, Pandya SB, Chaudhary KP, Padariya TJ, Alalawy MD, Patel MK, Dubey RP, Patel UH (2021) Coordination behavior of dinuclear silver complex of sulfamethoxazole with solvent molecule having static rotational disorder: spectroscopic characterization, crystal structure, Hirshfeld surface and antimicrobial activity. J Mol Struct 1228:129–777. https://doi.org/10.1016/j.molstruc.2020.129777
Dumond YR, Baker RL, Montchamp JL (2000) Orthosilicate-mediated esterification of monosubstituted phosphinic acids. Org Lett 21:3341–3344. https://doi.org/10.1021/ol006434g
Mathey F, Mercier F (1980) Conversion of phosphinic into phosphinous esters through nickelocene reduction–complexation of phosphinothioic O-esters. A new synthesis of carbon–phosphorus heterocycles. J.C.S. Chem Comm 4:191–192. https://doi.org/10.1039/C39800000191
Rodríguez VY, del Aguila MA, eIglesias MJ, Ortiz FL (2012) Directed ortho-lithiation of unprotected diphenylphosphinic acids. Tetrahedron 68:7355–7362. https://doi.org/10.1016/j.tet.2012.06.088
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Azzouni, S., Gaucher, A., Hassen, S. et al. From Phosphonic Acid to O–P Heterocycles Using MW: An Access to [5]-, [6]- and [8]-Membered Annelated Phosphinates. Chemistry Africa 7, 1187–1199 (2024). https://doi.org/10.1007/s42250-023-00816-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-023-00816-y