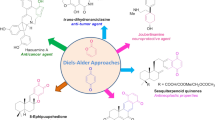
Abstract
Epoxy chalcone is a heterocyclic molecule and an important precursor for the synthesis of biologically active compounds. This mini-review is elucidating the synthetic strategies of chalcone epoxide via one pot and two pot routes including nature of reactants, nature of catalyst and variety of catalyst to improve yield of desired product, biological applications and advantages and drawbacks of these synthetic strategies. One pot route has wide variety of reactants and efficient one is the condensation of aldehyde and ketone. However, two pot route is consisted of chalcone synthesis preceding to epoxy chalcone. The synthetic routes for the preparation of epoxy chalcone and the usage as a precursor for the synthesis of various organic molecules with biological application is comprehensively discussed. This review is outstanding in organic chemistry and pharmaceutical industries to produce new molecules with various applications.
Graphic Abstract













Similar content being viewed by others
References
Umeda R, Muraki M, Nakamura Y et al (2017) Rhenium complex-catalyzed Meinwald rearrangement reactions of oxiranes. Tetrahedron Lett 58:2393–2395. https://doi.org/10.1016/j.tetlet.2017.05.018
Kumar S, Konduru NK, Verma N, Ahmed N (2015) β-cyclodextrin in water: highly versatile and green approach for biomimetic regioselective ring opening of chalcone epoxides with nitrogen heterocycles. Synth Commun 45:2555–2566. https://doi.org/10.1080/00397911.2015.1093142
Duan Y, Zhou B, Lin J-H, Xiao J-C (2015) Diastereoselective Johnson–Corey–Chaykovsky trifluoroethylidenation. Chem Commun 51:13127–13130. https://doi.org/10.1039/C5CC04991A
Riera A, Moreno M (2010) Synthetic applications of chiral unsaturated epoxy alcohols prepared by sharpless asymmetric epoxidation. Molecules 15:1041–1073. https://doi.org/10.3390/molecules15021041
Shaikh IR (2014) Organocatalysis: key trends in green synthetic chemistry, challenges, scope towards heterogenization, and importance from research and industrial point of view. J Catal 2014:1–35. https://doi.org/10.1155/2014/402860
Kuznetsov ML, Rocha BGM, Pombeiro AJL, Shul’pin GB (2015) Oxidation of olefins with hydrogen peroxide catalyzed by bismuth salts: a mechanistic study. ACS Catal 5:3823–3835. https://doi.org/10.1021/acscatal.5b00077
Hamerton I (1996) Recent developments in epoxy resins. iSmithers Rapra Publishing, Shrewsbury
McGarrigle EM, Gilheany DG (2005) Chromium- and manganese-salen promoted epoxidation of alkenes. Chem Rev 105:1563–1602. https://doi.org/10.1021/cr0306945
Rampa A, Bartolini M, Pruccoli L et al (2018) Exploiting the chalcone scaffold to develop multifunctional agents for Alzheimer’s disease. Molecules 23:1902. https://doi.org/10.3390/molecules23081902
Kakati D, Sarma RK, Saikia R et al (2013) Rapid microwave assisted synthesis and antimicrobial bioevaluation of novel steroidal chalcones. Steroids 78:321–326. https://doi.org/10.1016/j.steroids.2012.12.003
Farooq S, Ngaini Z (2020) One-pot and two-pot synthesis of chalcone based mono and bis-pyrazolines. Tetrahedron Lett 61:151416. https://doi.org/10.1016/j.tetlet.2019.151416
Wies S, Grynkiewicz G, Rusin A (2016) Isoflavones, their glycosides and glycoconjugates. synthesis and biological activity. Curr Org Chem 21:218–235. https://doi.org/10.2174/1385272820666160928120822
Rudrapal M, Satyanandam RS, Swaroopini TS et al (2013) Synthesis and antibacterial activity of some new hydrazones. Med Chem Res 22:2840–2846. https://doi.org/10.1007/s00044-012-0278-5
Aggarwal R, Kumar R (2009) Iodobenzene diacetate mediated oxidation of n-substituted hydrazones of chalcones: an efficient regioselective synthesis of 1,3,5-trisubstituted pyrazoles. Synth Commun 39:2169–2177. https://doi.org/10.1080/00397910802640038
Hammock, BD, Zheng J, Morisseau C, et al (2014) Inhibitors of epoxide hydrolases for the treatment of inflammation. US 8,815,951 B2 54
Mehanna AS (2014) Design, synthesis and calcium channel blocking activity of diltiazem-verapamil hybrid molecules. Med Chem 4:704–708. https://doi.org/10.4172/2161-0444.1000216
Ley SV, Tackett MN, Maddess ML et al (2009) Total synthesis of rapamycin. Chem Eur J 15:2874–2914. https://doi.org/10.1002/chem.200801656
Katukojvala S, Barlett KN, Lotesta SD, Williams LJ (2004) Spirodiepoxides in total synthesis: epoxomicin. J Am Chem Soc 126:15348–15349. https://doi.org/10.1021/ja044563c
Schmidt U, Schmidt J (1992) The synthesis of eponemycin. J Chem Soc Chem Commun 7:529–530
Pereira AR, Kale AJ, Fenley AT et al (2012) The carmaphycins: new proteasome inhibitors exhibiting an α, β-epoxyketone warhead from a marine cyanobacterium. ChemBioChem 13:810–817. https://doi.org/10.1002/cbic.201200007
Bhat BA, Puri SC, Qurishi MA et al (2005) Synthesis of 3,5-diphenyl-1 H-pyrazoles. Synth Commun 35:1135–1142. https://doi.org/10.1081/SCC-200054225
Fahmy AFM, El-Sayed AA, Hemdan MM et al (2017) Synthesis of N-containing heterocycles via mechanochemical grinding and conventional techniques. Asian J Chem 29:2679–2686. https://doi.org/10.14233/ajchem.2017.20798
Bhat BA, Dhar KL, Puri SC et al (2005) Synthesis and biological evaluation of chalcones and their derived pyrazoles as potential cytotoxic agents. Bioorg Med Chem Lett 15:3177–3180. https://doi.org/10.1016/j.bmcl.2005.03.121
Nair D, Pavashe P, Katiyar S, Namboothiri INN (2016) Regioselective synthesis of pyrazole and pyridazine esters from chalcones and α-diazo-β-ketoesters. Tetrahedron Lett 57:3146–3149. https://doi.org/10.1016/j.tetlet.2016.06.020
Ruan L, Shi M, Mao S et al (2014) An efficient approach to construct 2-arylbenzo[b]furans from 2-methoxychalcone epoxides. Tetrahedron 70:1065–1070. https://doi.org/10.1016/j.tet.2013.12.050
Vieira LCC, Matsuo BT, Martelli LSR et al (2017) Asymmetric synthesis of new γ-butenolides via organocatalyzed epoxidation of chalcones. Org Biomol Chem 15:6098–6103. https://doi.org/10.1039/C7OB00165G
Konduru NK, Ahmed N (2013) Regioselective opening of chalcone epoxides with nitrogen heterocycles using indium(iii) chloride as an efficient catalyst. Synth Commun 43:2008–2018. https://doi.org/10.1080/00397911.2012.667291
Li J-T, Sun M-X, Yin Y (2010) Ultrasound promoted efficient method for the cleavage of 3-aryl-2,3-epoxyl-1-phenyl-1-propanone with indole. Ultrason Sonochem 17:359–362. https://doi.org/10.1016/j.vultsonch.2009.09.004
Lu N, Zhang N, Zeng C-C et al (2015) Electrochemically induced ring-opening/friedel–crafts arylation of chalcone epoxides catalyzed by a triarylimidazole redox mediator. J Org Chem 80:781–789. https://doi.org/10.1021/jo5022184
Diez D, Nunez MG, Anton AB et al (2008) Asymmetric epoxidation of electron-deficient olefins. Curr Org Synth 5:186–216
Weiss KM, Tsogoeva SB (2011) Enantioselective epoxidation of electron-deficient olefins: an organocatalytic approach. Chem Rec 11:18–39
Zhu Y, Wang Q, Cornwall RG, Shi Y (2014) Organocatalytic asymmetric epoxidation and aziridination of olefins and their synthetic applications. Chem Rev 114:8199–8256. https://doi.org/10.1021/cr500064w
Wang C, Yamamoto H (2015) Asymmetric epoxidation using hydrogen peroxide as oxidant. Chem Asian J 10:2056–2068. https://doi.org/10.1002/asia.201500293
Aggarwal VK, Hynd G, Picoul W, Vasse J-L (2002) Highly enantioselective darzens reaction of a camphor-derived sulfonium amide to give glycidic amides and their applications in synthesis. J Am Chem Soc 124:9964–9965. https://doi.org/10.1021/ja0272540
Liu Y, Provencher BA, Bartelson KJ, Deng L (2011) Highly enantioselective asymmetric Darzens reactions with a phase transfer catalyst. Chem Sci 2:1301–1304. https://doi.org/10.1039/c1sc00137j
Nemcsok T, Rapi Z, Keglevich G et al (2018) Synthesis of D-mannitol-based crown ethers and their application as catalyst in asymmetric phase transfer reactions. Chirality 30:407–419. https://doi.org/10.1002/chir.22800
Rapi Z, Bakó P, Drahos L, Keglevich G (2015) Side-arm effect of a methyl α-d-glucopyranoside based lariat ether catalysts in asymmetric synthese. Heteroat Chem 26:63–71. https://doi.org/10.1002/hc.21214
Pálvölgyi Á, Rapi Z, Ozohanics O et al (2018) Synthesis of alkyl α- and β-d-glucopyranoside-based chiral crown ethers and their application as enantioselective phase-transfer catalysts. Res Chem Intermed 44:1627–1645. https://doi.org/10.1007/s11164-017-3189-8
Reddi RN, Prasad PK, Sudalai A (2015) N-heterocyclic carbene catalyzed oxidative coupling of alkenes/α-bromoacetophenones with aldehydes: a facile entry to α, β-epoxy ketones. Angew Chem Int Ed 54:14150–14153. https://doi.org/10.1002/anie.201507363
Li J, Wang DZ (2015) Visible-light-promoted photoredox syntheses of α, β-epoxy ketones from styrenes and benzaldehydes under alkaline conditions. Org Lett 17:5260–5263. https://doi.org/10.1021/acs.orglett.5b02629
Ashokkumar V, Siva A (2017) One-pot synthesis of α, β-epoxy ketones through domino reaction between alkenes and aldehydes catalyzed by proline based chiral organocatalysts. Org Biomol Chem 15:2551–2561. https://doi.org/10.1039/C7OB00031F
Xiang M, Ni X, Yi X et al (2015) Preparation of mesoporous zeolite ETS-10 catalysts for high-yield synthesis of α, β-epoxy ketones. ChemCatChem 7:521–525. https://doi.org/10.1002/cctc.201402839
Wei W-T, Yang X-H, Li H-B, Li J-H (2015) Oxidative coupling of alkenes with aldehydes and hydroperoxides: one-pot synthesis of 2,3-epoxy ketones. Adv Synth Catal 357:59–63. https://doi.org/10.1002/adsc.201400629
Chen S, Shao Z, Fang Z et al (2016) Design and synthesis of the basic Cu-doped zeolite X catalyst with high activity in oxidative coupling reactions. J Catal 338:38–46. https://doi.org/10.1016/j.jcat.2016.01.030
de Souza GFP, Bonacin JA, Salles AG (2018) Visible-light-driven epoxyacylation and hydroacylation of olefins using methylene blue/persulfate system in water. J Org Chem 83:8331–8340. https://doi.org/10.1021/acs.joc.8b01026
Liu W, Li Y, Liu K, Li Z (2011) Iron-catalyzed carbonylation-peroxidation of alkenes with aldehydes and hydroperoxides. J Am Chem Soc 133:10756–10759. https://doi.org/10.1021/ja204226n
Singh R, Kumar S, Singh KN (2017) One pot synthesis of α, β-epoxy ketones by oxidative coupling of methyl arenes with cinnamic acids involving C(sp 3)-H activation and decarboxylative strategy. Tetrahedron 73:3074–3078. https://doi.org/10.1016/j.tet.2017.04.025
Omura R, Fujiya A, Yamaguchi E et al (2016) One-pot aerobic photooxidative Darzens reaction from styrene and benzyl alcohol via phenacyl iodide and benzaldehyde by using-iodine. Synthesis 48:3971–3975. https://doi.org/10.1055/s-0035-1562455
Wang Y, Ye J, Liang X (2007) Convenient preparation of chiral α, β-epoxy ketones via Claisen–Schmidt condensation-epoxidation sequence. Adv Synth Catal 349:1033–1036. https://doi.org/10.1002/adsc.200600592
Luo W, Yu Z, Qiu W et al (2011) One-pot production of chiral α, β-epoxy ketones from benzaldehydes and acetophenones by recyclable poly(amino acid) catalysis. Tetrahedron 67:5289–5292. https://doi.org/10.1016/j.tet.2011.05.024
Crivoi D-G, Segarra AM, Medina F (2016) Highly selective multifunctional nanohybrid catalysts for the one-pot synthesis of α, β-epoxy-chalcones. J Catal 334:120–128. https://doi.org/10.1016/j.jcat.2015.11.020
Choudary BM, Kantam ML, Ranganath KVS et al (2004) Bifunctional nanocrystalline MgO for chiral epoxy ketones via Claisen–Schmidt condensation–asymmetric epoxidation reactions. J Am Chem Soc 126:3396–3397. https://doi.org/10.1021/ja038954n
Mahato S, Santra S, De A et al (2018) A domino approach for the synthesis of α, β-epoxy ketones from carbonyl compounds under neat conditions at ambient temperature. ChemistrySelect 3:7596–7601. https://doi.org/10.1002/slct.201801162
Ngo D, Kalala M, Hogan V, Manchanayakage R (2014) One-pot synthesis of chalcone epoxides—a green chemistry strategy. Tetrahedron Lett 55:4496–4500. https://doi.org/10.1016/j.tetlet.2014.06.057
Dai L-Z, Shi M (2009) A convenient and efficient one-pot way to synthesize α, β-epoxy ketones directly from acetophenones and arylaldehydes. Tetrahedron Lett 50:651–655. https://doi.org/10.1016/j.tetlet.2008.11.110
Manchanayakage R (2016) One-pot synthesis of chalcone epoxides via Claisen Schmidt condensation and epoxidation. In: ACS symposium series “in green chemistry experiments in undergraduate laboratories”, pp 111–122
Ngaini Z, Norashikin Irdawaty AR (2010) Synthesis and characterization of cyclotriphosphazenes bearing chalcones derivatives. Phosphorus Sulfur Silicon 185:628–633
Porter MJ, Skidmore J (2009) Asymmetric epoxidation of electron-deficient alkenes. In: Denmark SE et al (eds) Organic reactions. Wiley, Hoboken, pp 426–672
Hussain H, Al-Harrasi A, Green IR et al (2014) Meta-chloroperbenzoic acid (m-CPBA): a versatile: reagent in organic synthesis. RSC Adv 4:12882–12917
Fioroni G, Fringuelli F, Pizzo F, Vaccaro L (2003) Epoxidation of α, β-unsaturated ketones in water. An environmentally benign protocol. Green Chem 5:425–428. https://doi.org/10.1039/B303883A
Jew S, Park H (2009) Cinchona-based phase-transfer catalysts for asymmetric synthesis. Chem Commun. https://doi.org/10.1039/b914028j
Singh G, Yeboah E (2016) Recent applications of Cinchona alkaloid-based catalysts in asymmetric addition reactions. Rep Org Chem 6:47–75. https://doi.org/10.2147/ROC.S73908
Kacprzak K, Gawroński J (2004) Cinchona alkaloids and their derivatives: versatile catalysts and ligands in asymmetric synthesis. Synthesis 2001:961–998. https://doi.org/10.1055/s-2001-14560
Farooq S, Ngaini Z (2019) Recent synthetic methodologies for chalcone synthesis (2013–2018). Curr Organocatal 06:1–9. https://doi.org/10.2174/2213337206666190306155140
Abbasi-Dehnavi H, Ghashang M (2018) Solvent-free preparation of 3-aryl-2-[(aryl)(arylamino)]methyl-4H-furo[3,2-c]chromen-4-one derivatives using ZnO-ZnAl2O4 nanocomposite as a heterogeneous catalyst. Heterocycl Commun 24:19–22. https://doi.org/10.1515/hc-2017-0141
Lyakin OY, Bryliakov KP, Talsi EP (2019) Non-heme oxoiron(V) intermediates in chemo-, regio- and stereoselective oxidation of organic substrates. Coord Chem Rev 384:126–139. https://doi.org/10.1016/j.ccr.2019.01.010
Cui L, Furuhashi S, Tachikawa Y et al (2013) Efficient generation of hydrogen peroxide by aerobic photooxidation of 2-propanol using anthraquinone-2-carboxylic acid and one-pot epoxidation of α, β-unsaturated ketones. Tetrahedron Lett 54:162–165. https://doi.org/10.1016/j.tetlet.2012.10.119
Nagano M, Doi M, Kurihara M et al (2010) Stabilized α-helix-catalyzed enantioselective epoxidation of α, β-unsaturated ketones. Org Lett 12:3564–3566. https://doi.org/10.1021/ol101435w
Victor NJ, Gana J, Muraleedharan KM (2015) N-methylpyrrolidone hydroperoxide/Cs2CO3 as an excellent reagent system for the hydroxy-directed diastereoselective epoxidation of electron-deficient olefins. Chem Eur J 21:14742–14747. https://doi.org/10.1002/chem.201501929
Pielichowski J, Kowalski G (2010) Novel polyaniline supported cobalt catalyzed aerobic oxidation of unsaturated organic compounds. Mol Cryst Liq Cryst 522:105–111. https://doi.org/10.1080/15421401003719829
Shen D, Miao C, Wang S et al (2014) A mononuclear manganese complex of a tetradentate nitrogen ligand—synthesis, characterizations, and application in the asymmetric epoxidation of olefins: bioinspired manganese complex for olefin epoxidation. Eur J Inorg Chem 2014:5777–5782. https://doi.org/10.1002/ejic.201402663
Mphahlele MJ, Maluleka MM, Mampa RM (2019) Elucidation of the structure of the 2-amino-3,5-dibromochalcone epoxides in solution and solid state. Crystals 9:277. https://doi.org/10.3390/cryst9060277
Huo C, An J, Xu X et al (2013) Tandem reaction between chalcone epoxides and 2-naphthyl ethers to construct complex naphtho[2,1-b]furans. Tetrahedron Lett 54:1145–1148. https://doi.org/10.1016/j.tetlet.2012.12.078
Guerra J, Cantillo D, Kappe CO (2016) Visible-light photoredox catalysis using a macromolecular ruthenium complex: reactivity and recovery by size-exclusion nanofiltration in continuous flow. Catal Sci Technol 6:4695–4699. https://doi.org/10.1039/C6CY00070C
Hasegawa E, Izumiya N, Fukuda T et al (2016) Visible light-promoted reductive transformations of various organic substances by using hydroxyaryl-substituted benzimidazolines and bases. Tetrahedron 72:7805–7812. https://doi.org/10.1016/j.tet.2016.05.078
Hasegawa E, Arai S, Tayama E, Iwamoto H (2015) Metal-free, one-pot, sequential protocol for transforming α, β-epoxy ketones to β-hydroxy ketones and α-methylene ketones. J Org Chem 80:1593–1600. https://doi.org/10.1021/jo5025249
Osisanya RA, Oluwadiya JO (1989) Synthesis of N-heterocycles via chalcone epoxides. 1. Amino and hydrazinopyrimidines. J Heterocycl Chem 26:947–948. https://doi.org/10.1002/jhet.5570260412
Ponra S, Majumdar KC (2016) Brønsted acid-promoted synthesis of common heterocycles and related bio-active and functional molecules. RSC Adv 6:37784–37922. https://doi.org/10.1039/C5RA27069C
Brasil SR, Capiotto ADC, Flores AFC et al (2017) Unexpected formation of bis(hydrazinecarboximidamide) via ultrasound promoted rearrangement of epoxy ketone. Orbital Electron J Chem 9:204–209. https://doi.org/10.17807/orbital.v9i3.972
Yang Z, Jiang B, Hao W-J et al (2014) Synthesis of enaminones and their difluoroboron complexes through domino aryl migration. Chem Commun 51:1267–1270
Tu M-S, Xu H-W, Fan W et al (2015) [4 + 2] heterocyclization for efficient formation of substituted quinoxalines through carbon-oxygen bonds cleavage: efficient formation of quinoxalines. J Heterocycl Chem 52:719–725. https://doi.org/10.1002/jhet.2128
Mahato S, Chatterjee R, Santra S et al (2017) A domino approach for the synthesis of α-iodo-β-dicarbonyl compounds from α-epoxycarbonyls. ChemistrySelect 2:6254–6259. https://doi.org/10.1002/slct.201700867
Szeja W, Grynkiewicz G, Rusin A (2017) Isoflavones, their glycosides and glycoconjugates. Synthesis and biological activity. Curr Org Chem 21:218–235
Verma N, Kumar S, Ahmed N (2017) LiBr/β-CD/IBX/H2O-DMSO: a new approach for one-pot biomimetic regioselective ring opening of chalcone epoxides to bromohydrins and conversion to 1,2,3-triketones. Synth Commun 47:1110–1120. https://doi.org/10.1080/00397911.2017.1315537
Jadhav BG, Vaidya AA, Samant SD (2015) Copper(II)triflate promoted highly chemoselective rearrangement of chalcone epoxides to β-keto aldehydes. Lett Org Chem 12:55–61
Khalaj M, Taherkhani M, Naderi F, Mousavi-Safavi SM (2018) Catalytic multicomponent reaction between nitroalkanes, elemental sulfur, and oxiranes. Monatshefte Für Chem Chem Mon 149:63–71. https://doi.org/10.1007/s00706-017-2067-9
Caneva T, Sperni L, Strukul G, Scarso A (2016) Efficient epoxide isomerization within a self-assembled hexameric organic capsule. RSC Adv 6:83505–83509
Marais JPJ, Ferreira D, Slade D (2005) Stereoselective synthesis of monomeric flavonoids. Phytochemistry 66:2145–2176. https://doi.org/10.1016/j.phytochem.2005.03.006
Tanaka K, Toda F (2000) Solvent-free organic synthesis. Chem Rev 100:1025–1074. https://doi.org/10.1021/cr940089p
Yamaguchi K, Mori K, Mizugaki T et al (2000) Epoxidation of α, β-unsaturated ketones using hydrogen peroxide in the presence of basic hydrotalcite catalysts. J Org Chem 65:6897–6903. https://doi.org/10.1021/jo000247e
Baars S, Drauz K-H, Krimmer H-P et al (2003) Development of the Juliá–Colonna asymmetric epoxidation reaction: part 1. preparation and activation of the polyleucine catalyst. Org Process Res Dev 7:509–513. https://doi.org/10.1021/op0300037
Bakó T, Bakó P, Keglevich G et al (2004) Phase-transfer catalyzed asymmetric epoxidation of chalcones using chiral crown ethers derived from d-glucose, d-galactose, and d-mannitol. Tetrahedron Asymmetry 15:1589–1595. https://doi.org/10.1016/j.tetasy.2004.03.029
Kakei H, Tsuji R, Ohshima T, Shibasaki M (2005) Catalytic asymmetric epoxidation of α, β-unsaturated esters using an yttrium-biphenyldiol complex. J Am Chem Soc 127:8962–8963. https://doi.org/10.1021/ja052466t
Hemingway RW, Laks PE (1992) Plant polyphenols. Springer, Boston
Helder R, Hummelen JC, Laane R et al (1976) Catalytic asymmetric induction in oxidation reactions. The synthesis of optically active epoxides. Tetrahedron Lett 17:1831–1834
Mandal T, Jana S, Dash J (2017) Zinc-mediated efficient and selective reduction of carbonyl compounds: zinc-mediated efficient and selective reduction of carbonyl compounds. Eur J Org Chem 2017:4972–4983. https://doi.org/10.1002/ejoc.201700887
Wang H, Ren S, Zhang J et al (2015) Selectfluor-mediated simultaneous cleavage of C–O and C–C Bonds in α, β-epoxy ketones under transition-metal-free conditions: a route to 1,2-diketones. J Org Chem 80:6856–6863. https://doi.org/10.1021/acs.joc.5b00857
Ke Q, Zhang B, Hu B et al (2014) A transition-metal-free, one-pot procedure for the synthesis of α, β-epoxy ketones by oxidative coupling of alkenes and aldehydes via base catalysis. Chem Commun 51:1012–1015. https://doi.org/10.1039/C4CC09260K
Ji-Tai L, Xian-Feng L, Yin Y, Du C (2009) Synthesis of 2, 3-epoxy-1-phenyl-3-aryl-1-propanone by combination of phase transfer catalyst and ultrasound irradiation. Org Commun 2:1–9
Lattanzi A, Cocilova M, Iannece P, Scettri A (2004) Enantioselective epoxidation of chalcones and naphthoquinones mediated by (+)-norcamphor-derived hydroperoxide. Tetrahedron Asymmetry 15:3751–3755. https://doi.org/10.1016/j.tetasy.2004.10.009
Mahmoodi NO, Yazdanbakhsh MR, Ghanbari F (2010) Epoxidation of 1,4-diaroyl ethene derivatives in the presence of UHP or H2O2. Synth Commun 40:3181–3185. https://doi.org/10.1080/00397910903372333
Colonna S, Manfredi A (1986) Catalytic asymmetric Weitz–Scheffer reaction in the presence of bovine serum albumin. Tetrahedron Lett 27:387–390. https://doi.org/10.1016/S0040-4039(00)84026-5
Jin H, Zhao H, Zhao F et al (2009) Efficient epoxidation of chalcones with urea-hydrogen peroxide under ultrasound irradiation. Ultrason Sonochem 16:304–307. https://doi.org/10.1016/j.ultsonch.2008.10.013
Allingham MT, Bennett EL, Davies DH et al (2016) Synthesis, applications and mechanistic investigations of C2 symmetric guanidinium salts. Tetrahedron 72:496–503. https://doi.org/10.1016/j.tet.2015.11.058
Bérubé C, Voyer N (2018) Crown-ether-modified cyclic dipeptides as supramolecular chiral catalysts. Supramol Chem 30:184–195. https://doi.org/10.1080/10610278.2017.1392521
Ottenbacher RV, Samsonenko DG, Talsi EP, Bryliakov KP (2016) Enantioselective epoxidations of olefins with various oxidants on bioinspired Mn complexes: evidence for different mechanisms and chiral additive amplification. ACS Catal 6:979–988. https://doi.org/10.1021/acscatal.5b02299
Chen X, Gao B, Su Y, Huang H (2017) Enantioselective epoxidation of electron-deficient alkenes catalyzed by manganese complexes with chiral N4 ligands derived from rigid chiral diamines. Adv Synth Catal 359:2535–2541. https://doi.org/10.1002/adsc.201700541
Miao C, Yan X, Xu D et al (2017) Bioinspired manganese complexes and graphene oxide synergistically catalyzed asymmetric epoxidation of olefins with aqueous hydrogen peroxide. Adv Synth Catal 359:476–484. https://doi.org/10.1002/adsc.201600848
Qian Q, Tan Y, Zhao B et al (2014) Asymmetric epoxidation of unsaturated ketones catalyzed by heterobimetallic rare earth-lithium complexes bearing phenoxy-functionalized chiral diphenylprolinolate ligand. Org Lett 16:4516–4519. https://doi.org/10.1021/ol5020398
Kulkarni NV, Das A, Ridlen SG et al (2016) Fluorinated triazapentadienyl ligand supported ethyl zinc(II) complexes: reaction with dioxygen and catalytic applications in the Tishchenko reaction. Dalton Trans 45:4896–4906. https://doi.org/10.1039/C6DT00257A
Kubisiak M, Zelga K, Justyniak I et al (2013) Catalytic epoxidation of enones mediated by zinc alkylperoxide tert-BuOOH systems. Organometallics 32:5263–5265. https://doi.org/10.1021/om400830e
Raheem Keeri A, Justyniak I, Jurczak J, Lewiński J (2016) Quest for efficient catalysts based on zinc tert-butyl peroxides for asymmetric epoxidation of enones: C2- vs C1-symmetric auxiliaries. Adv Synth Catal 358:864–868. https://doi.org/10.1002/adsc.201500764
Rulev YA, Larionov VA, Lokutova AV et al (2016) Chiral cobalt(III) complexes as bifunctional brønsted acid-lewis base catalysts for the preparation of cyclic organic carbonates. Chemsuschem 9:216–222. https://doi.org/10.1002/cssc.201501365
Larionov VA, Markelova EP, Smol’yakov AF et al (2015) Chiral octahedral complexes of Co(III) as catalysts for asymmetric epoxidation of chalcones under phase transfer conditions. RSC Adv 5:72764–72771. https://doi.org/10.1039/C5RA11760G
Cruchter T, Larionov VA (2018) Asymmetric catalysis with octahedral stereogenic-at-metal complexes featuring chiral ligands. Coord Chem Rev 376:95–113. https://doi.org/10.1016/j.ccr.2018.08.002
Talsi EP, Rybalova TV, Bryliakov KP (2016) Ti-salalen mediated asymmetric epoxidation of olefins with H2O2: effect of ligand on the catalytic performance, and insight into the oxidation mechanism. J Mol Catal Chem 421:131–137. https://doi.org/10.1016/j.molcata.2016.05.019
Zima AM, Lyakin OY, Ottenbacher RV et al (2016) Dramatic effect of carboxylic acid on the electronic structure of the active species in Fe(PDP)-catalyzed asymmetric epoxidation. ACS Catal 6:5399–5404. https://doi.org/10.1021/acscatal.6b01473
Tabatabaeian K, Zanjanchi MA, NosratO M et al (2017) Diimino nickel complex anchored into the MOF cavity as catalyst for epoxidation of chalcones and bischalcones. J Clust Sci 28:949–962. https://doi.org/10.1007/s10876-016-1079-7
Jarzyński S, Utecht G, Leśniak S, Rachwalski M (2017) Highly enantioselective asymmetric reactions involving zinc ions promoted by chiral aziridine alcohols. Tetrahedron Asymmetry 28:1774–1779. https://doi.org/10.1016/j.tetasy.2017.10.007
Bérubé C, Voyer N (2016) Biomimetic epoxidation in aqueous media catalyzed by cyclic dipeptides. Synth Commun 46:395–403. https://doi.org/10.1080/00397911.2016.1141428
Bérubé C, Barbeau X, Cardinal S et al (2017) Interfacial supramolecular biomimetic epoxidation catalysed by cyclic dipeptides. Supramol Chem 29:330–349. https://doi.org/10.1080/10610278.2016.1236197
Bérubé C, Barbeau X, Lagüe P, Voyer N (2017) Revisiting the Juliá–Colonna enantioselective epoxidation: supramolecular catalysis in water. Chem Commun 53:5099–5102. https://doi.org/10.1039/C7CC01168G
Ardila-Fierro KJ, Crawford DE, Körner A et al (2018) Papain-catalysed mechanochemical synthesis of oligopeptides by milling and twin-screw extrusion: application in the Juliá–Colonna enantioselective epoxidation. Green Chem 20:1262–1269. https://doi.org/10.1039/C7GC03205F
Ramananarivo HR, Maati H, Amadine O et al (2015) Ecofriendly synthesis of ceria foam via carboxymethylcellulose gelation: application for the epoxidation of chalcone. ACS Sustain Chem Eng 3:2786–2795. https://doi.org/10.1021/acssuschemeng.5b00662
Sela T, Lin X, Vigalok A (2017) Concentrated aqueous sodium tosylate as green medium for alkene oxidation and nucleophilic substitution reactions. J Org Chem 82:11609–11612. https://doi.org/10.1021/acs.joc.7b01679
Wang P, Cai J, Yang J et al (2013) Solvent-dependent regioselective oxidation of trans-chalcones using aqueous hydrogen peroxide. J Braz Chem Soc 24:518–522. https://doi.org/10.5935/0103-5053.20130063
Saffar-Teluri A (2016) Bovine bone derived natural nanocrystalline hydroxyapatite supported boron trifluoride: an efficient, recyclable, and eco-friendly heterogeneous catalyst for diastereoselective formation of α-hydroxy-β-methoxyketones. Synth React Inorg, Met Org, Nano-Met Chem 46:83–86. https://doi.org/10.1080/15533174.2014.900638
Khosravi K, Naserifar S (2017) Facile epoxidation of α, β-unsaturated ketones with urea-2,2-dihydroperoxypropane as a new oxidant. J Iran Chem Soc 14:323–328. https://doi.org/10.1007/s13738-016-0980-1
Khosravi K, Naserifar S, Mahmoudi B (2017) Metal-free and efficient epoxidation of α, β-unsaturated ketones with 1, 1, 2, 2-tetrahydroperoxy-1, 2-diphenylethane as a powerful solid oxidant. J Chin Chem Soc 64:683–689
Wu Y, Zhou G, Meng Q et al (2018) Visible light-induced aerobic epoxidation of α, β-unsaturated ketones mediated by amidines. J Org Chem 83:13051–13062. https://doi.org/10.1021/acs.joc.8b01710
Farooq S, Munawar MA, Ngaini Z (2019) Two pot and one pot synthetic methodologies of Hantzsch pyridines. Curr Org Chem 22:2671–2680. https://doi.org/10.2174/1385272822666181109124547
Zhuang C, Zhang W, Sheng C et al (2017) Chalcone: a privileged structure in medicinal chemistry. Chem Rev 117:7762–7810. https://doi.org/10.1021/acs.chemrev.7b00020
Vachon J, Lacour J (2006) Recent developments in enantioselective phase transfer catalysis using chiral ammonium salts. Chim Int J Chem 60:266–275
Umaa K, Krishnakumar K, Dineshkumar B, Kannan K (2013) Synthesis, characterization and antifungal evaluation of acetyl 2-methylbenzimidazolyl amino derivatives. Int J Chem Sci Technol 3:9–14
Han H, Zhao Y, Cuthbertson T et al (2010) Cell cycle arrest and apoptosis induction by an anticancer chalcone epoxide. Arch Pharm (Weinheim) 343:429–439. https://doi.org/10.1002/ardp.200900261
Daraei B, Karimi G, Makhdoumi P, Zarghi A (2017) Evaluation of cytotoxicity effects of chalcone epoxide analogues as a selective COX-II inhibitor in the human liver carcinoma cell line. J Pharmacopuncture 20:207–212. https://doi.org/10.3831/KPI.2017.20.024
Mirzaei H, Emami S (2016) Recent advances of cytotoxic chalconoids targeting tubulin polymerization: synthesis and biological activity. Eur J Med Chem 121:610–639. https://doi.org/10.1016/j.ejmech.2016.05.067
El-Meligie S, Taher AT, Kamal AM, Youssef A (2017) Design, synthesis and cytotoxic activity of certain novel chalcone analogous compounds. Eur J Med Chem 126:52–60. https://doi.org/10.1016/j.ejmech.2016.09.099
De Simone G, Di Fiore A, Capasso C, Supuran CT (2015) The zinc coordination pattern in the η-carbonic anhydrase from Plasmodium falciparum is different from all other carbonic anhydrase genetic families. Bioorg Med Chem Lett 25:1385–1389. https://doi.org/10.1016/j.bmcl.2015.02.046
Casey DA, Antimisiaris D, O’Brien J (2010) Drugs for Alzheimer’s disease: are they effective? P T Peer-Rev J Formul Manag 35:208–211
Stellenboom N (2019) Comparison of the inhibitory potential towards carbonic anhydrase, acetylcholinesterase and butyrylcholinesterase of chalcone and chalcone epoxide: STELLENBOOM. J Biochem Mol Toxicol 33:e22240. https://doi.org/10.1002/jbt.22240
Acknowledgements
The authors would like to thank to Minister of Higher Education Malaysia and Postgraduate Research Grant, Universiti Malaysia Sarawak for financial support.
Funding
This work was supported by Minister of Higher Education through F07/FRGS/1883/2019 and Universiti Malaysia Sarawak through F07/PGRS/1794/2019.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Farooq, S., Ngaini, Z. One Pot and Two Pot Synthetic Strategies and Biological Applications of Epoxy-Chalcones. Chemistry Africa 3, 291–302 (2020). https://doi.org/10.1007/s42250-020-00128-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-020-00128-5