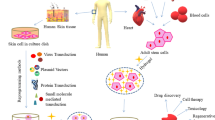
Abstract
Recent developments in organoid culture technologies have made it possible to closely recapitulate intrinsic characteristics of different tissues under in vitro conditions. These organoids act as a translational bridge between the traditional 2D/3D cultures and the in vivo models for studying the tissue development processes, disease modeling, and drug screening. Matrigel and tissue-specific extracellular matrix have been shown to support organoid development, efficiently; however, their chemically undefined nature, non-tunable properties, and associated batch-to-batch variations often limit reproducibility of the assembly process. In this regard, chemically defined platforms offer wider opportunities to optimize and recreate tissue-specific microenvironment. The present review delineates the current research trends in this sphere, focusing on material perspective and the target tissues (e.g., neural, liver, pancreatic, renal, and intestinal). The review winds up with a discussion on the current limitations and future perspective to provide a basis for future research.



Reproduced with permission from Ref. [94]. Copyright © 2016, Ranga et al.

Reproduced with permission from Ref. [101]. Copyright © 2020, Sorrentino et al.

Reproduced with permission from Ref. [75]. Copyright © 2020, Ye et al.

Reproduced with permission from Ref. [56]. Copyright © 2020, Georgakopoulos et al.

Reproduced with permission from Ref. [59]. Copyright © 2017, Elsevier B. V.

Reproduced with permission from Ref. [74]. Copyright © 2020 Elsevier B. V.

Reproduced with permission from Ref. [52]. Copyright © 2018, Broguiere et al. Published by WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim

Reproduced with permission from Ref. [74]. Copyright © 2020, Elsevier B. V.

Similar content being viewed by others
Abbreviations
- 2D:
-
Two dimensional
- 3D:
-
Three dimensional
- SD:
-
Standard deviation
- ECM:
-
Extracellular matrix
- EHS:
-
Engelbreth–Holm–Swarm
- LMN:
-
Laminin
- COLIV:
-
Collagen IV
- HA:
-
Hyaluronic acid
- PEG:
-
Polyethylene glycol
- PIC:
-
Polyisocyanopeptide
- MMPs:
-
Matrix metalloproteinases
- GLDH:
-
Glutamate dehydrogenase
- ESCs:
-
Embryonic stem cells
- HSPCs:
-
Hematopoietic stem and progenitor cells
- BMSCs:
-
Bone marrow stromal cells
- PEGDA:
-
Poly(ethylene glycol) diacrylate
- MSC:
-
Mesenchymal stem cells
- FN:
-
Fibronectin
- DV:
-
Dorsal–ventral
- iPSCs:
-
Induced pluripotent stem cells
- ALB:
-
Albumin
- MDR1:
-
Multidrug resistance protein 1
- CNF:
-
Cellulose nanofibril
- ALAT:
-
Alanine aminotransferase
- ASAT:
-
Aspartate transaminase
- PEGDE:
-
Poly(ethylene glycol) diglycidyl ether
- LGR5:
-
Leucine-rich repeat-containing G-protein-coupled receptor 5
- BME2:
-
Basement Membrane Extract Type 2
- dPMP:
-
Degradable PEG-MMP-PEG
- ndPH:
-
Non-degradable PEG–heparin
- dPMH:
-
Degradable PEG-MMP-Heparin
- LEC:
-
LMN–entactin complex
- HNF4α:
-
Hepatocyte nuclear factor 4α
- E-cad:
-
E-cadherin
- KRT19:
-
Cytokeratin 19
- NHE3:
-
Sodium–hydrogen exchanger 3
- Muc2:
-
Mucin 2
- Lyz:
-
Lysozyme
References
Huch M, Koo B-K (2015) Modeling mouse and human development using organoid cultures. Development 142:3113–3125. https://doi.org/10.1242/dev.118570
Kaushik G, Ponnusamy MP, Batra SK (2018) Concise review: current status of three-dimensional organoids as preclinical models. Stem Cells 36:1329–1340. https://doi.org/10.1002/stem.2852
Kretzschmar K, Clevers H (2016) Organoids: modeling development and the stem cell niche in a dish. Dev Cell 38:590–600. https://doi.org/10.1016/j.devcel.2016.08.014
Kim J, Koo B-K, Knoblich JA (2020) Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol 21:571–584. https://doi.org/10.1038/s41580-020-0259-3
Dedhia PH, Bertaux-Skeirik N, Zavros Y, Spence JR (2016) Organoid models of human gastrointestinal development and disease. Gastroenterology 150:1098–1112. https://doi.org/10.1053/j.gastro.2015.12.042
Pastuła A, Middelhoff M, Brandtner A, Tobiasch M, Höhl B, Nuber AH, Demir IE, Neupert S, Kollmann P, Mazzuoli-Weber G, Quante M (2016) Three-dimensional gastrointestinal organoid culture in combination with nerves or fibroblasts: a method to characterize the gastrointestinal stem cell niche. Stem Cells Int 2016:1–16. https://doi.org/10.1155/2016/3710836
Kim G-A, Spence JR, Takayama S (2017) Bioengineering for intestinal organoid cultures. Curr Opin Biotechnol 47:51–58. https://doi.org/10.1016/j.copbio.2017.05.006
Takahashi Y, Takebe T, Taniguchi H (2018) Methods for generating vascularized islet-like organoids via self-condensation. Curr Protoc Stem Cell Biol 45:e49. https://doi.org/10.1002/cpsc.49
Abdal Dayem A, Bin LS, Kim K, Lim KM, Jeon T, Cho S-G (2019) Recent advances in organoid culture for insulin production and diabetes therapy: methods and challenges. BMB Rep 52:295–303. https://doi.org/10.5483/BMBRep.2019.52.5.089
Hu H, Gehart H, Artegiani B, Löpez-Iglesias C, Dekkers F, Basak O, van Es J, de Sousa Chuva, Lopes SM, Begthel H, Korving J, van den Born M, Zou C, Quirk C, Chiriboga L, Rice CM, Ma S, Rios A, Peters PJ, de Jong YP, Clevers H (2018) Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell 175:1591-1606.e19. https://doi.org/10.1016/j.cell.2018.11.013
Asai A, Aihara E, Watson C, Mourya R, Mizuochi T, Shivakumar P, Phelan K, Mayhew C, Helmrath M, Takebe T, Wells J, Bezerra JA (2017) Paracrine signals regulate human liver organoid maturation from induced pluripotent stem cells. Development 144:1056–1064. https://doi.org/10.1242/dev.142794
Morizane R, Bonventre JV (2017) Kidney organoids: a translational journey. Trends Mol Med 23:246–263. https://doi.org/10.1016/j.molmed.2017.01.001
Kumar SV, Er PX, Lawlor KT, Motazedian A, Scurr M, Ghobrial I, Combes AN, Zappia L, Oshlack A, Stanley EG, Little MH (2019) Kidney micro-organoids in suspension culture as a scalable source of human pluripotent stem cell-derived kidney cells. Development 146:dev172361. https://doi.org/10.1242/dev.172361
Boretto M, Cox B, Noben M, Hendriks N, Fassbender A, Roose H, Amant F, Timmerman D, Tomassetti C, Vanhie A, Meuleman C, Ferrante M, Vankelecom H (2017) Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development 144:1775–1786. https://doi.org/10.1242/dev.148478
Fitzgerald HC, Dhakal P, Behura SK, Schust DJ, Spencer TE (2019) Self-renewing endometrial epithelial organoids of the human uterus. Proc Natl Acad Sci 116:23132–23142. https://doi.org/10.1073/pnas.1915389116
Nugraha B, Buono MF, Boehmer L, Hoerstrup SP, Emmert MY (2019) Human cardiac organoids for disease modeling. Clin Pharmacol Ther 105:79–85. https://doi.org/10.1002/cpt.1286
Hoang P, Wang J, Conklin BR, Healy KE, Ma Z (2018) Generation of spatial-patterned early-developing cardiac organoids using human pluripotent stem cells. Nat Protoc 13:723–737. https://doi.org/10.1038/nprot.2018.006
Llonch S, Carido M, Ader M (2018) Organoid technology for retinal repair. Dev Biol 433:132–143. https://doi.org/10.1016/j.ydbio.2017.09.028
Völkner M, Zschätzsch M, Rostovskaya M, Overall RW, Busskamp V, Anastassiadis K, Karl MO (2016) Retinal organoids from pluripotent stem cells efficiently recapitulate retinogenesis. Stem Cell Reports 6:525–538. https://doi.org/10.1016/j.stemcr.2016.03.001
Sachs N, Papaspyropoulos A, Zomer-van Ommen DD, Heo I, Böttinger L, Klay D, Weeber F, Huelsz-Prince G, Iakobachvili N, Amatngalim GD, Ligt J, Hoeck A, Proost N, Viveen MC, Lyubimova A, Teeven L, Derakhshan S, Korving J, Begthel H, Dekkers JF, Kumawat K, Ramos E, Oosterhout MF, Offerhaus GJ, Wiener DJ, Olimpio EP, Dijkstra KK, Smit EF, Linden M, Jaksani S, Ven M, Jonkers J, Rios AC, Voest EE, Moorsel CH, Ent CK, Cuppen E, Oudenaarden A, Coenjaerts FE, Meyaard L, Bont LJ, Peters PJ, Tans SJ, Zon JS, Boj SF, Vries RG, Beekman JM, Clevers H (2019) Long-term expanding human airway organoids for disease modeling. EMBO J 38:100300. https://doi.org/10.15252/embj.2018100300
Tan Q, Choi KM, Sicard D, Tschumperlin DJ (2017) Human airway organoid engineering as a step toward lung regeneration and disease modeling. Biomaterials 113:118–132. https://doi.org/10.1016/j.biomaterials.2016.10.046
Poznansky MC, Evans RH, Foxall RB, Olszak IT, Piascik AH, Hartman KE, Brander C, Meyer TH, Pykett MJ, Chabner KT, Kalams SA, Rosenzweig M, Scadden DT (2000) Efficient generation of human T cells from a tissue-engineered thymic organoid. Nat Biotechnol 18:729–734. https://doi.org/10.1038/77288
Bar-Ephraim YE, Kretzschmar K, Clevers H (2020) Organoids in immunological research. Nat Rev Immunol 20:279–293. https://doi.org/10.1038/s41577-019-0248-y
Kelava I, Lancaster MA (2016) Dishing out mini-brains: Current progress and future prospects in brain organoid research. Dev Biol 420:199–209. https://doi.org/10.1016/j.ydbio.2016.06.037
Heide M, Huttner WB, Mora-Bermúdez F (2018) Brain organoids as models to study human neocortex development and evolution. Curr Opin Cell Biol 55:8–16. https://doi.org/10.1016/j.ceb.2018.06.006
Lei M, Schumacher LJ, Lai Y-C, Juan W-T, Yeh C-Y, Wu P, Jiang T-X, Baker RE, Widelitz RB, Yang L, Chuong C-M (2017) Self-organization process in newborn skin organoid formation inspires strategy to restore hair regeneration of adult cells. Proc Natl Acad Sci 114:E7101–E7110. https://doi.org/10.1073/pnas.1700475114
Kim Y, Ju JH (2019) Generation of 3D skin organoid from cord blood-derived induced pluripotent stem cells. J Vis Exp. https://doi.org/10.3791/59297
Aisenbrey EA, Murphy WL (2020) Synthetic alternatives to Matrigel. Nat Rev Mater 5:539–551. https://doi.org/10.1038/s41578-020-0199-8
Blondel D, Lutolf MP (2019) Bioinspired hydrogels for 3D organoid culture. Chim Int J Chem 73:81–85. https://doi.org/10.2533/chimia.2019.81
Holloway EM, Capeling MM, Spence JR (2019) Biologically inspired approaches to enhance human organoid complexity. Development 146:dev166173. https://doi.org/10.1242/dev.166173
Jin Y, Kim J, Lee JS, Min S, Kim S, Ahn D-H, Kim Y-G, Cho S-W (2018) Vascularized liver organoids generated using induced hepatic tissue and dynamic liver-specific microenvironment as a drug testing platform. Adv Funct Mater 28:1801954. https://doi.org/10.1002/adfm.201801954
Hun M, Barsanti M, Wong K, Ramshaw J, Werkmeister J, Chidgey AP (2017) Native thymic extracellular matrix improves in vivo thymic organoid T cell output, and drives in vitro thymic epithelial cell differentiation. Biomaterials 118:1–15. https://doi.org/10.1016/j.biomaterials.2016.11.054
Mollica PA, Booth-Creech EN, Reid JA, Zamponi M, Sullivan SM, Palmer X-L, Sachs PC, Bruno RD (2019) 3D bioprinted mammary organoids and tumoroids in human mammary derived ECM hydrogels. Acta Biomater 95:201–213. https://doi.org/10.1016/j.actbio.2019.06.017
Bi H, Karanth SS, Ye K, Stein R, Jin S (2020) Decellularized tissue matrix enhances self-assembly of islet organoids from pluripotent stem cell differentiation. ACS Biomater Sci Eng 6:4155–4165. https://doi.org/10.1021/acsbiomaterials.0c00088
Magno V, Meinhardt A, Werner C (2020) Polymer hydrogels to guide organotypic and organoid cultures. Adv Funct Mater 30:2000097. https://doi.org/10.1002/adfm.202000097
Giobbe GG, Crowley C, Luni C, Campinoti S, Khedr M, Kretzschmar K, De Santis MM, Zambaiti E, Michielin F, Meran L, Hu Q, van Son G, Urbani L, Manfredi A, Giomo M, Eaton S, Cacchiarelli D, Li VSW, Clevers H, Bonfanti P, Elvassore N, De Coppi P (2019) Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat Commun 10:5658. https://doi.org/10.1038/s41467-019-13605-4
Agarwal T, Narayan R, Maji S, Ghosh SK, Maiti TK (2018) Decellularized caprine liver extracellular matrix as a 2D substrate coating and 3D hydrogel platform for vascularized liver tissue engineering. J Tissue Eng Regen Med 12:e1678–e1690. https://doi.org/10.1002/term.2594
Kaukonen R, Jacquemet G, Hamidi H, Ivaska J (2017) Cell-derived matrices for studying cell proliferation and directional migration in a complex 3D microenvironment. Nat Protoc 12:2376–2390. https://doi.org/10.1038/nprot.2017.107
Ventre M, Netti P (2016) Controlling cell functions and fate with surfaces and hydrogels: the role of material features in cell adhesion and signal transduction. Gels 2:12. https://doi.org/10.3390/gels2010012
Chen AA, Thomas DK, Ong LL, Schwartz RE, Golub TR, Bhatia SN (2011) Humanized mice with ectopic artificial liver tissues. Proc Natl Acad Sci 108:11842–11847. https://doi.org/10.1073/pnas.1101791108
Li J, Chen Y, Kawazoe N, Chen G (2018) Ligand density-dependent influence of arginine–glycine–aspartate functionalized gold nanoparticles on osteogenic and adipogenic differentiation of mesenchymal stem cells. Nano Res 11:1247–1261. https://doi.org/10.1007/s12274-017-1738-5
Meyer M (2019) Processing of collagen based biomaterials and the resulting materials properties. Biomed Eng Online 18:24. https://doi.org/10.1186/s12938-019-0647-0
Echave MC, Burgo LS, Pedraz JL, Orive G (2017) Gelatin as biomaterial for tissue engineering. Curr Pharm Des 23:3567–3584. https://doi.org/10.2174/0929867324666170511123101
Huang W, Navarro-Serer B, Jeong YJ, Chianchiano P, Xia L, Luchini C, Veronese N, Dowiak C, Ng T, Trujillo MA, Huang B, Pflüger MJ, Macgregor-Das AM, Lionheart G, Jones D, Fujikura K, Nguyen-Ngoc K-V, Neumann NM, Groot VP, Hasanain A, van Oosten AF, Fischer SE, Gallinger S, Singhi AD, Zureikat AH, Brand RE, Gaida MM, Heinrich S, Burkhart RA, He J, Wolfgang CL, Goggins MG, Thompson ED, Roberts NJ, Ewald AJ, Wood LD (2020) Pattern of invasion in human pancreatic cancer organoids is associated with loss of SMAD4 and clinical outcome. Cancer Res 80:2804–2817. https://doi.org/10.1158/0008-5472.CAN-19-1523
Capeling M, Huang S, Mulero-Russe A, Cieza R, Tsai Y-H, Garcia A, Hill DR (2020) Generation of small intestinal organoids for experimental intestinal physiology. Methods Cell Biol 159:143–174. https://doi.org/10.1016/bs.mcb.2020.03.007
Sachs N, Tsukamoto Y, Kujala P, Peters PJ, Clevers H (2017) Intestinal epithelial organoids fuse to form self-organizing tubes in floating collagen gels. Development 144:1107–1112. https://doi.org/10.1242/dev.143933
Jabaji Z, Brinkley GJ, Khalil HA, Sears CM, Lei NY, Lewis M, Stelzner M, Martín MG, Dunn JCY (2014) Type I collagen as an extracellular matrix for the in vitro growth of human small intestinal epithelium. PLoS ONE 9:e107814. https://doi.org/10.1371/journal.pone.0107814
Sun W, Incitti T, Migliaresi C, Quattrone A, Casarosa S, Motta A (2016) Genipin-crosslinked gelatin–silk fibroin hydrogels for modulating the behaviour of pluripotent cells. J Tissue Eng Regen Med 10:876–887. https://doi.org/10.1002/term.1868
Broderick EP, O’Halloran DM, Rochev YA, Griffin M, Collighan RJ, Pandit AS (2005) Enzymatic stabilization of gelatin-based scaffolds. J Biomed Mater Res 72B:37–42. https://doi.org/10.1002/jbm.b.30119
Zhang YS, Pi Q, van Genderen AM (2017) Microfluidic bioprinting for engineering vascularized tissues and organoids. J Vis Exp. https://doi.org/10.3791/55957
Ahmed TAE, Dare EV, Hincke M (2008) Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev 14:199–215. https://doi.org/10.1089/ten.teb.2007.0435
Broguiere N, Isenmann L, Hirt C, Ringel T, Placzek S, Cavalli E, Ringnalda F, Villiger L, Züllig R, Lehmann R, Rogler G, Heim MH, Schüler J, Zenobi-Wong M, Schwank G (2018) Growth of epithelial organoids in a defined hydrogel. Adv Mater 30:1801621. https://doi.org/10.1002/adma.201801621
Wang Y, Liu H, Zhang M, Wang H, Chen W, Qin J (2020) One-step synthesis of composite hydrogel capsules to support liver organoid generation from hiPSCs. Biomater Sci 8:5476–5488. https://doi.org/10.1039/D0BM01085E
Collins MN, Birkinshaw C (2013) Hyaluronic acid based scaffolds for tissue engineering—a review. Carbohydr Polym 92:1262–1279. https://doi.org/10.1016/j.carbpol.2012.10.028
Khunmanee S, Jeong Y, Park H (2017) Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J Tissue Eng 8:204173141772646. https://doi.org/10.1177/2041731417726464
Georgakopoulos N, Prior N, Angres B, Mastrogiovanni G, Cagan A, Harrison D, Hindley CJ, Arnes-Benito R, Liau S-S, Curd A, Ivory N, Simons BD, Martincorena I, Wurst H, Saeb-Parsy K, Huch M (2020) Long-term expansion, genomic stability and in vivo safety of adult human pancreas organoids. BMC Dev Biol 20:4. https://doi.org/10.1186/s12861-020-0209-5
Liang Y, Kiick KL (2014) Heparin-functionalized polymeric biomaterials in tissue engineering and drug delivery applications. Acta Biomater 10:1588–1600. https://doi.org/10.1016/j.actbio.2013.07.031
Schirmer L, Chwalek K, Tsurkan MV, Freudenberg U, Werner C (2020) Glycosaminoglycan-based hydrogels with programmable host reactions. Biomaterials 228:119557. https://doi.org/10.1016/j.biomaterials.2019.119557
Weber HM, Tsurkan MV, Magno V, Freudenberg U, Werner C (2017) Heparin-based hydrogels induce human renal tubulogenesis in vitro. Acta Biomater 57:59–69. https://doi.org/10.1016/j.actbio.2017.05.035
Nowak M, Freudenberg U, Tsurkan MV, Werner C, Levental KR (2017) Modular GAG-matrices to promote mammary epithelial morphogenesis in vitro. Biomaterials 112:20–30. https://doi.org/10.1016/j.biomaterials.2016.10.007
Sun J, Tan H (2013) Alginate-based biomaterials for regenerative medicine applications. Materials (Basel) 6:1285–1309. https://doi.org/10.3390/ma6041285
Agarwal T, Kabiraj P, Narayana GH, Kulanthaivel S, Kasiviswanathan U, Pal K, Giri S, Maiti TK, Banerjee I (2016) Alginate bead based hexagonal close packed 3D implant for bone tissue engineering. ACS Appl Mater Interfaces 8:32132–32145. https://doi.org/10.1021/acsami.6b08512
Kulanthaivel S, Rathnam VSS, Agarwal T, Pradhan S, Pal K, Giri S, Maiti TK, Banerjee I (2017) Gum tragacanth–alginate beads as proangiogenic–osteogenic cell encapsulation systems for bone tissue engineering. J Mater Chem B 5:4177–4189. https://doi.org/10.1039/C7TB00390K
Capeling MM, Czerwinski M, Huang S, Tsai Y-H, Wu A, Nagy MS, Juliar B, Sundaram N, Song Y, Han WM, Takayama S, Alsberg E, Garcia AJ, Helmrath M, Putnam AJ, Spence JR (2019) Nonadhesive alginate hydrogels support growth of pluripotent stem cell-derived intestinal organoids. Stem Cell Reports 12:381–394. https://doi.org/10.1016/j.stemcr.2018.12.001
Agarwal T, Narayan R, Maji S, Behera S, Kulanthaivel S, Maiti TK, Banerjee I, Pal K, Giri S (2016) Gelatin/carboxymethyl chitosan based scaffolds for dermal tissue engineering applications. Int J Biol Macromol 93:1499–1506. https://doi.org/10.1016/j.ijbiomac.2016.04.028
Croisier F, Jérôme C (2013) Chitosan-based biomaterials for tissue engineering. Eur Polym J 49:780–792. https://doi.org/10.1016/j.eurpolymj.2012.12.009
Lindborg BA, Brekke JH, Vegoe AL, Ulrich CB, Haider KT, Subramaniam S, Venhuizen SL, Eide CR, Orchard PJ, Chen W, Wang Q, Pelaez F, Scott CM, Kokkoli E, Keirstead SA, Dutton JR, Tolar J, O’Brien TD (2016) Rapid induction of cerebral organoids from human induced pluripotent stem cells using a chemically defined hydrogel and defined cell culture medium. Stem Cells Transl Med 5:970–979. https://doi.org/10.5966/sctm.2015-0305
Argüelles-Monal W, Lizardi-Mendoza J, Fernández-Quiroz D, Recillas-Mota M, Montiel-Herrera M (2018) Chitosan derivatives: introducing new functionalities with a controlled molecular architecture for innovative materials. Polymers (Basel) 10:342. https://doi.org/10.3390/polym10030342
Zhang Y, Tang C, Span PN, Rowan AE, Aalders TW, Schalken JA, Adema GJ, Kouwer PHJ, Zegers MMP, Ansems M (2020) Polyisocyanide hydrogels as a tunable platform for mammary gland organoid formation. Adv Sci 7:2001797. https://doi.org/10.1002/advs.202001797
Unal AZ, West JL (2020) Synthetic ECM: Bioactive synthetic hydrogels for 3D tissue engineering. Bioconjug Chem 31:2253–2271. https://doi.org/10.1021/acs.bioconjchem.0c00270
D’souza AA, Shegokar R, (2016) Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expert Opin Drug Deliv 13:1257–1275. https://doi.org/10.1080/17425247.2016.1182485
Chen G, Tang W, Wang X, Zhao X, Chen C, Zhu Z (2019) Applications of hydrogels with special physical properties in biomedicine. Polymers (Basel) 11:1420. https://doi.org/10.3390/polym11091420
Cruz-Acuña R, Quirós M, Farkas AE, Dedhia PH, Huang S, Siuda D, García-Hernández V, Miller AJ, Spence JR, Nusrat A, García AJ (2017) Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat Cell Biol 19:1326–1335. https://doi.org/10.1038/ncb3632
Hernandez-Gordillo V, Kassis T, Lampejo A, Choi G, Gamboa ME, Gnecco JS, Brown A, Breault DT, Carrier R, Griffith LG (2020) Fully synthetic matrices for in vitro culture of primary human intestinal enteroids and endometrial organoids. Biomaterials 254:120125. https://doi.org/10.1016/j.biomaterials.2020.120125
Ye S, Boeter JWB, Mihajlovic M, Steenbeek FG, Wolferen ME, Oosterhoff LA, Marsee A, Caiazzo M, Laan LJW, Penning LC, Vermonden T, Spee B, Schneeberger K (2020) A chemically defined hydrogel for human liver organoid culture. Adv Funct Mater 30:2000893. https://doi.org/10.1002/adfm.202000893
Zimoch J, Padial JS, Klar AS, Vallmajo-Martin Q, Meuli M, Biedermann T, Wilson CJ, Rowan A, Reichmann E (2018) Polyisocyanopeptide hydrogels: A novel thermo-responsive hydrogel supporting pre-vascularization and the development of organotypic structures. Acta Biomater 70:129–139. https://doi.org/10.1016/j.actbio.2018.01.042
Gu L, Shan T, Ma Y, Tay FR, Niu L (2019) Novel biomedical applications of crosslinked collagen. Trends Biotechnol 37:464–491. https://doi.org/10.1016/j.tibtech.2018.10.007
Sorushanova A, Delgado LM, Wu Z, Shologu N, Kshirsagar A, Raghunath R, Mullen AM, Bayon Y, Pandit A, Raghunath M, Zeugolis DI (2019) The collagen suprafamily: from biosynthesis to advanced biomaterial development. Adv Mater 31:1801651. https://doi.org/10.1002/adma.201801651
Van Hoorick J, Tytgat L, Dobos A, Ottevaere H, Van Erps J, Thienpont H, Ovsianikov A, Dubruel P, Van Vlierberghe S (2019) (Photo-)crosslinkable gelatin derivatives for biofabrication applications. Acta Biomater 97:46–73. https://doi.org/10.1016/j.actbio.2019.07.035
Wang X, Ao Q, Tian X, Fan J, Tong H, Hou W, Bai S (2017) Gelatin-based hydrogels for organ 3D bioprinting. Polymers (Basel) 9:401. https://doi.org/10.3390/polym9090401
de Melo BAG, Jodat YA, Cruz EM, Benincasa JC, Shin SR, Porcionatto MA (2020) Strategies to use fibrinogen as bioink for 3D bioprinting fibrin-based soft and hard tissues. Acta Biomater 117:60–76. https://doi.org/10.1016/j.actbio.2020.09.024
Sakiyama-Elbert SE (2014) Incorporation of heparin into biomaterials. Acta Biomater 10:1581–1587. https://doi.org/10.1016/j.actbio.2013.08.045
Salwowska NM, Bebenek KA, Żądło DA, Wcisło-Dziadecka DL (2016) Physiochemical properties and application of hyaluronic acid: a systematic review. J Cosmet Dermatol 15:520–526. https://doi.org/10.1111/jocd.12237
Pereira H, Sousa DA, Cunha A, Andrade R, Espregueira-Mendes J, Oliveira JM, Reis RL (2018) Hyaluronic acid. In: Osteochondral tissue engineering. Advances in experimental medicine and biology, vol 1059. pp 137–153. https://doi.org/10.1007/978-3-319-76735-2_6
Rastogi P, Kandasubramanian B (2019) Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication 11:042001. https://doi.org/10.1088/1758-5090/ab331e
Vanacker J, Amorim CA (2017) Alginate: a versatile biomaterial to encapsulate isolated ovarian follicles. Ann Biomed Eng 45:1633–1649. https://doi.org/10.1007/s10439-017-1816-6
Lee KY, Mooney DJ (2012) Alginate: Properties and biomedical applications. Prog Polym Sci 37:106–126. https://doi.org/10.1016/j.progpolymsci.2011.06.003
Muxika A, Etxabide A, Uranga J, Guerrero P, de la Caba K (2017) Chitosan as a bioactive polymer: processing, properties and applications. Int J Biol Macromol 105:1358–1368. https://doi.org/10.1016/j.ijbiomac.2017.07.087
Muanprasat C, Chatsudthipong V (2017) Chitosan oligosaccharide: biological activities and potential therapeutic applications. Pharmacol Ther 170:80–97. https://doi.org/10.1016/j.pharmthera.2016.10.013
Cruz-Acuña R, Quirós M, Huang S, Siuda D, Spence JR, Nusrat A, García AJ (2018) PEG-4MAL hydrogels for human organoid generation, culture, and in vivo delivery. Nat Protoc 13:2102–2119. https://doi.org/10.1038/s41596-018-0036-3
Gjorevski N, Lutolf MP (2017) Synthesis and characterization of well-defined hydrogel matrices and their application to intestinal stem cell and organoid culture. Nat Protoc 12:2263–2274. https://doi.org/10.1038/nprot.2017.095
Vallmajo-Martin Q, Broguiere N, Millan C, Zenobi-Wong M, Ehrbar M (2020) PEG/HA hybrid hydrogels for biologically and mechanically tailorable bone marrow organoids. Adv Funct Mater 30:1910282. https://doi.org/10.1002/adfm.201910282
Wechsler ME, Shevchuk M, Peppas NA (2020) Developing a multidisciplinary approach for engineering stem cell organoids. Ann Biomed Eng 48:1895–1904. https://doi.org/10.1007/s10439-019-02391-1
Ranga A, Girgin M, Meinhardt A, Eberle D, Caiazzo M, Tanaka EM, Lutolf MP (2016) Neural tube morphogenesis in synthetic 3D microenvironments. Proc Natl Acad Sci 113:E6831–E6839. https://doi.org/10.1073/pnas.1603529113
Balion Z, Cėpla V, Svirskiene N, Svirskis G, Druceikaitė K, Inokaitis H, Rusteikaitė J, Masilionis I, Stankevičienė G, Jelinskas T, Ulčinas A, Samanta A, Valiokas R, Jekabsone A (2020) Cerebellar cells self-assemble into functional organoids on synthetic, chemically crosslinked ECM-mimicking peptide hydrogels. Biomolecules 10:754. https://doi.org/10.3390/biom10050754
Agarwal T, Subramanian B, Maiti TK (2019) Liver tissue engineering: challenges and opportunities. ACS Biomater Sci Eng 5:4167–4182. https://doi.org/10.1021/acsbiomaterials.9b00745
Miyaoka Y, Ebato K, Kato H, Arakawa S, Shimizu S, Miyajima A (2012) Hypertrophy and unconventional cell division of hepatocytes underlie liver regeneration. Curr Biol 22:1166–1175. https://doi.org/10.1016/j.cub.2012.05.016
Xu H, Jiao Y, Qin S, Zhao W, Chu Q, Wu K (2018) Organoid technology in disease modelling, drug development, personalized treatment and regeneration medicine. Exp Hematol Oncol 7:30. https://doi.org/10.1186/s40164-018-0122-9
Underhill GH, Khetani SR (2018) Bioengineered liver models for drug testing and cell differentiation studies. Cell Mol Gastroenterol Hepatol 5:426-439.e1. https://doi.org/10.1016/j.jcmgh.2017.11.012
Akbari S, Arslan N, Senturk S, Erdal E (2019) Next-generation liver medicine using organoid models. Front Cell Dev Biol 7:345. https://doi.org/10.3389/fcell.2019.00345
Sorrentino G, Rezakhani S, Yildiz E, Nuciforo S, Heim MH, Lutolf MP, Schoonjans K (2020) Mechano-modulatory synthetic niches for liver organoid derivation. Nat Commun 11:3416. https://doi.org/10.1038/s41467-020-17161-0
Krüger M, Oosterhoff LA, van Wolferen ME, Schiele SA, Walther A, Geijsen N, De Laporte L, van der Laan LJW, Kock LM, Spee B (2020) Cellulose nanofibril hydrogel promotes hepatic differentiation of human liver organoids. Adv Healthc Mater 9:1901658. https://doi.org/10.1002/adhm.201901658
Hohwieler M, Müller M, Frappart P-O, Heller S (2019) Pancreatic progenitors and organoids as a prerequisite to model pancreatic diseases and cancer. Stem Cells Int 2019:1–11. https://doi.org/10.1155/2019/9301382
Greggio C, De Franceschi F, Figueiredo-Larsen M, Gobaa S, Ranga A, Semb H, Lutolf M, Grapin-Botton A (2013) Artificial three-dimensional niches deconstruct pancreas development in vitro. Development 140:4452–4462. https://doi.org/10.1242/dev.096628
Candiello J, Grandhi TSP, Goh SK, Vaidya V, Lemmon-Kishi M, Eliato KR, Ros R, Kumta PN, Rege K, Banerjee I (2018) 3D heterogeneous islet organoid generation from human embryonic stem cells using a novel engineered hydrogel platform. Biomaterials 177:27–39. https://doi.org/10.1016/j.biomaterials.2018.05.031
Ding B, Sun G, Liu S, Peng E, Wan M, Chen L, Jackson J, Atala A (2020) Three-dimensional renal organoids from whole kidney cells: generation, optimization, and potential application in nephrotoxicology in vitro. Cell Transplant 29:096368971989706. https://doi.org/10.1177/0963689719897066
Webster AC, Nagler EV, Morton RL, Masson P (2017) Chronic kidney disease. Lancet 389:1238–1252. https://doi.org/10.1016/S0140-6736(16)32064-5
Garreta E, Montserrat N, Belmonte JCI (2018) Kidney organoids for disease modeling. Oncotarget 9:12552–12553. https://doi.org/10.18632/oncotarget.24438
Enemchukwu NO, Cruz-Acuña R, Bongiorno T, Johnson CT, García JR, Sulchek T, García AJ (2016) Synthetic matrices reveal contributions of ECM biophysical and biochemical properties to epithelial morphogenesis. J Cell Biol 212:113–124. https://doi.org/10.1083/jcb.201506055
Cruz-Acuña R, Mulero-Russe A, Clark AY, Zent R, García AJ (2019) Identification of matrix physicochemical properties required for renal epithelial cell tubulogenesis by using synthetic hydrogels. J Cell Sci 132:jcs226639. https://doi.org/10.1242/jcs.226639
Costa J, Ahluwalia A (2019) Advances and current challenges in intestinal in vitro model engineering: a digest. Front Bioeng Biotechnol 7:144. https://doi.org/10.3389/fbioe.2019.00144
Qi D, Shi W, Black AR, Kuss MA, Pang X, He Y, Liu B, Duan B (2020) Repair and regeneration of small intestine: a review of current engineering approaches. Biomaterials 240:119832. https://doi.org/10.1016/j.biomaterials.2020.119832
Sato T, Stange DE, Ferrante M, Vries RGJ, van Es JH, van den Brink S, van Houdt WJ, Pronk A, van Gorp J, Siersema PD, Clevers H (2011) Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141:1762–1772. https://doi.org/10.1053/j.gastro.2011.07.050
DiMarco RL, Dewi RE, Bernal G, Kuo C, Heilshorn SC (2015) Protein-engineered scaffolds for in vitro 3D culture of primary adult intestinal organoids. Biomater Sci 3:1376–1385. https://doi.org/10.1039/C5BM00108K
Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, Ordóñez-Morán P, Clevers H, Lutolf MP (2016) Designer matrices for intestinal stem cell and organoid culture. Nature 539:560–564. https://doi.org/10.1038/nature20168
Florian S, Iwamoto Y, Coughlin M, Weissleder R, Mitchison TJ (2019) A human organoid system that self-organizes to recapitulate growth and differentiation of a benign mammary tumor. Proc Natl Acad Sci 116:11444–11453. https://doi.org/10.1073/pnas.1702372116
Gu Z-Y, Jia S-Z, Liu S, Leng J-H (2020) Endometrial organoids: a new model for the research of endometrial-related diseases. Biol Reprod 103:918–926. https://doi.org/10.1093/biolre/ioaa124
Graney PL, Lai K, Post S, Brito I, Cyster J, Singh A (2020) Organoid polymer functionality and mode of Klebsiella pneumoniae membrane antigen presentation regulates ex vivo germinal center epigenetics in young and aged B cells. Adv Funct Mater 30:2001232. https://doi.org/10.1002/adfm.202001232
Jansen LE, McCarthy TP, Lee MJ, Peyton SR (2018) A synthetic, three-dimensional bone marrow hydrogel. bioRxiv 275842. https://doi.org/10.1101/275842
Bejoy J, Wang Z, Bijonowski B, Yang M, Ma T, Sang Q-X, Li Y (2018) Differential effects of heparin and hyaluronic acid on neural patterning of human induced pluripotent stem cells. ACS Biomater Sci Eng 4:4354–4366. https://doi.org/10.1021/acsbiomaterials.8b01142
Ng S, Tan WJ, Pek MMX, Tan M-H, Kurisawa M (2019) Mechanically and chemically defined hydrogel matrices for patient-derived colorectal tumor organoid culture. Biomaterials 219:119400. https://doi.org/10.1016/j.biomaterials.2019.119400
DiMarco RL, Su J, Yan KS, Dewi R, Kuo CJ, Heilshorn SC (2014) Engineering of three-dimensional microenvironments to promote contractile behavior in primary intestinal organoids. Integr Biol 6:127–142. https://doi.org/10.1039/C3IB40188J
Barry C, Schmitz MT, Propson NE, Hou Z, Zhang J, Nguyen BK, Bolin JM, Jiang P, McIntosh BE, Probasco MD, Swanson S, Stewart R, Thomson JA, Schwartz MP, Murphy WL (2017) Uniform neural tissue models produced on synthetic hydrogels using standard culture techniques. Exp Biol Med 242:1679–1689. https://doi.org/10.1177/1535370217715028
Meinhardt A, Eberle D, Tazaki A, Ranga A, Niesche M, Wilsch-Bräuninger M, Stec A, Schackert G, Lutolf M, Tanaka EM (2014) 3D Reconstitution of the patterned neural tube from embryonic stem cells. Stem Cell Reports 3:987–999. https://doi.org/10.1016/j.stemcr.2014.09.020
Yavitt FM, Brown TE, Hushka EA, Brown ME, Gjorevski N, Dempsey PJ, Lutolf MP, Anseth KS (2020) The effect of thiol structure on allyl sulfide photodegradable hydrogels and their application as a degradable scaffold for organoid passaging. Adv Mater 32:1905366. https://doi.org/10.1002/adma.201905366
Bergenheim F, Fregni G, Buchanan CF, Riis LB, Heulot M, Touati J, Seidelin JB, Rizzi SC, Nielsen OH (2020) A fully defined 3D matrix for ex vivo expansion of human colonic organoids from biopsy tissue. Biomaterials 262:120248. https://doi.org/10.1016/j.biomaterials.2020.120248
Klotz BJ, Oosterhoff LA, Utomo L, Lim KS, Vallmajo-Martin Q, Clevers H, Woodfield TBF, Rosenberg AJWP, Malda J, Ehrbar M, Spee B, Gawlitta D (2019) A versatile biosynthetic hydrogel platform for engineering of tissue analogues. Adv Healthc Mater 8:1900979. https://doi.org/10.1002/adhm.201900979
Astashkina AI, Mann BK, Prestwich GD, Grainger DW (2012) A 3-D organoid kidney culture model engineered for high-throughput nephrotoxicity assays. Biomaterials 33:4700–4711. https://doi.org/10.1016/j.biomaterials.2012.02.063
Tian YF, Ahn H, Schneider RS, Yang SN, Roman-Gonzalez L, Melnick AM, Cerchietti L, Singh A (2015) Integrin-specific hydrogels as adaptable tumor organoids for malignant B and T cells. Biomaterials 73:110–119. https://doi.org/10.1016/j.biomaterials.2015.09.007
Kaylan KB, Ermilova V, Yada RC, Underhill GH (2016) Combinatorial microenvironmental regulation of liver progenitor differentiation by Notch ligands. TGFβ and extracellular matrix Sci Rep 6:23490. https://doi.org/10.1038/srep23490
Kourouklis AP, Kaylan KB, Underhill GH (2016) Substrate stiffness and matrix composition coordinately control the differentiation of liver progenitor cells. Biomaterials 99:82–94. https://doi.org/10.1016/j.biomaterials.2016.05.016
Kloxin AM, Kasko AM, Salinas CN, Anseth KS (2009) Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science (80-) 324:59–63.https://doi.org/10.1126/science.1169494
Rosales AM, Anseth KS (2016) The design of reversible hydrogels to capture extracellular matrix dynamics. Nat Rev Mater 1:15012. https://doi.org/10.1038/natrevmats.2015.12
Mohamed MA, Fallahi A, El-Sokkary AMA, Salehi S, Akl MA, Jafari A, Tamayol A, Fenniri H, Khademhosseini A, Andreadis ST, Cheng C (2019) Stimuli-responsive hydrogels for manipulation of cell microenvironment: from chemistry to biofabrication technology. Prog Polym Sci 98:101147. https://doi.org/10.1016/j.progpolymsci.2019.101147
Li X, Su X (2018) Multifunctional smart hydrogels: potential in tissue engineering and cancer therapy. J Mater Chem B 6:4714–4730. https://doi.org/10.1039/C8TB01078A
Acknowledgements
TA would like to acknowledge the INSPIRE scheme, Department of Science and Technology, Government of India, for providing the fellowship. Graphical abstract was created with BioRender.com. Authors would like to acknowledge the efforts of Ms. Sampriti Pal, Department of Biotechnology, Indian Institute of Technology Kharagpur, India, in proofreading the manuscript.
Author information
Authors and Affiliations
Contributions
TA contributed to conceptualization, writing—original draft, and writing—reviewing and editing; NC and MC performed writing—original draft; TKM contributed to conceptualization and writing—reviewing and editing; PM helped in writing–reviewing and editing.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest among themselves or with any funding agency.
Ethical approval
This article does not contain any studies with human or animal subjects performed by any of the authors.
Rights and permissions
About this article
Cite this article
Agarwal, T., Celikkin, N., Costantini, M. et al. Recent advances in chemically defined and tunable hydrogel platforms for organoid culture. Bio-des. Manuf. 4, 641–674 (2021). https://doi.org/10.1007/s42242-021-00126-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42242-021-00126-7