Abstract
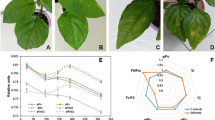
Fire blight caused by the gram-negative bacterium Erwinia amylovora is the most destructive disease of pome fruit trees. The pathogen induces the disease by secreting HrpN, HrpW, and DspA/E effector proteins into host tissues triggering oxidative burst. To study the role of these effectors in the course of interaction with host plants, hrpN−, hrpW− and dspA/E− mutants were inoculated on two pear cultivars under in situ (greenhouse and immature fruit) and in vitro systems. In addition, the determinantal role of these effectors on host chloroplasts was studied under activated and inactivated electron cascade of chloroplasts. Results showed that the lack of HrpN and DspA/E effectors postponed the initiation of necrosis and also decreased necrosis progress rate in comparison with the wild type strain. In contrast, hrpW− had the least decline in the pathogenicity of fire blight. The comparison of chloroplast activity confirmed the role of effectors in in situ experiments and also revealed that HrpN could be the main factor for the interaction of the pathogen with host cell chloroplasts.






Similar content being viewed by others
References
Abdollahi H, Ghahremani Z (2011) The role of chloroplasts in the Erwinia amylovora and the host plants. Acta Hortic 896:215–221
Abdollahi H, Rugini E, Ruzzi M, Muleo R (2004) In vitro system for studying the interaction between Erwinia amylovora and genotypes of pear. Plant Cell Tiss Org 79:90–95
Abdollahi H, Ghahremani Z, Erfani Nia K (2015) Role of electron transport chain of chloroplasts in oxidative burst of interaction between Erwinia amylovora and host cells. Photosynth Res 124:231–242
Bellemann P, Geider K (1992) Localization of transposon insertions in pathogenicity mutants of Erwinia amylovora and their biochemical characterization. J Gen Microbiol 138:931–940
Bhattacharjee S (2011) Sites of generation and physicochemical basis of formation of reactive oxygen species in plant cell in: DuttaGupta, S (Ed) reactive oxygen species and antioxidants in higher plants (pp 1-30) CRC press: Boca Raton
Boccara M, Schwartz W, Guiot E, Vidal G, De Paepe R, Dubois A, Boccara AC (2007) Early Chloroplastic alterations analyzed by optical coherence tomography during a harpin–induced hypersensitive response. Plant J 50:338–346
Bogdanove AJ, Bauer DW, Beer SV (1998a) Erwinia amylovora secretes DspE, a pathogenicity factor and functional AvrE homolog, through the Hrp (type III secretion) pathway. J Bacteriol 180:2244–2247
Bogdanove AJ, Kim JF, Wei ZM, Kolchinsky I, Charkowski AO, Conlin AK, Collmer A, Beer SV (1998b) Homology and functional similarity of a hrp–linked pathogenicity locus, dspEF, of Erwinia amylovora and the avirulence locus avrE of Pseudomonas syringae pathovar tomato. PNAS USA 95:1325–1330
Bonasera JM, Meng X, Owens T, Beer SV (2006) Interaction of DspE/a, a pathogenicity/avriulence protein of Erwinia amylovora, with pre–ferredoxin from apple and its relationship to photosynthetic efficiency. Acta Hortic 704:473–477
Boureau T, El Maarouf-Boutea H, Garnier A, Brisset MN, Perino C, Pucheu I, Barny MA (2006) DspA/E, a type III effector essential for Erwinia amylovora pathogenicity and growth in planta, induces cell death in host apple and non–host tobacco plants. Mol Plant-Microbe Interact 12:312–329
DebRoy S, Thilmony R, Kwack YB, Nomura K, He SY (2004) A family of conserved bacterial effectors inhibits salicylic acid–mediated basal immunity and promotes disease necrosis in plants. PNAS USA 101:9927–9932
Degrave A, Fagard M, Perino S, Brisset MN, Gaubert S, Laroche S, Patrit O, Barny MA (2008) Erwinia amylovora type three–secreted proteins trigger cell death and defence responses in Arabidopsis thaliana. Mol Plant-Microbe Interact 21:1076–1086
Dong H, Delaney T, Bauer D, Beer S (1999) Harpin induces disease resistance in Arabidopsis through the systemic acquired resistance pathway mediated by salicylic acid and the NIM1 gene. Plant J 20:207–215
Erfaninia K, Abdollahi H, Khosroshahi M (2014) Effect of several chloroplast electron transport chain inhibitors on interaction of Erwinia amylovora with susceptible and tolerant apple and pear cultivars. Plant Pathol 49:201–214 (In Farsi)
Gaudriault S, Malandrin L, Paulin JP, Barny MA (1997) DspA, an essential pathogenicity factor of Erwinia amylovora showing homology with AvrE of Pseudomonas syringae, is secreted via Hrp secretion pathway in a DspB–dependent way. Mol Microbiol 26:1057–1069
Khan MA, Zhao YF, Korban SS (2012) Molecular mechanisms of pathogenesis and resistance to the bacterial pathogen Erwinia amylovora, causal agent of fire blight disease in Rosaceae. Plant Mol Biol Rep 30:247–260
Kim JF, Beer SV (1998) HrpW of Erwinia amylovora, a new harpin that contains a domain homologous to pectatelyases of a distinct class. J Bacteriol 180:5203–5210
Menggad M, Laurent J (1998) Mutations in ams genes of Erwinia amylovora affect the interactions with host plants. Eur J Plant Pathol 104:313–322
Oh CS, Beer SV (2005) Molecular genetics of Erwinia amylovora involved in the development of fire blight. FEMS Microbiol Lett 253:185–192
Paret ML, deSilva AS, Criley RA, Alvarez AM (2008) Ralstonia solanacearum race 4: risk assessment for edible ginger floricultural ginger industries in Hawaii. Hort Technol 18:90–96
Percival GC, Henderson A (2003) An assessment of the freezing tolerance of urban trees using chlorophyll fluorescence. J Hortic Sci Biotechnol 78:254–260
Quoirin M, Lepoivre P (1977) Etude de milieux adaptes aux cultures in vitro de Prunus. Acta Hortic 78:437–442
Salehi Z (2016) Evaluation of salicylic acid effects on tolerance to the biotic and abiotic stresses in apple and pears dissertation, Azad University of Karaj, Iran
Steinberger EM, Cheng GY, Beer SV (1990) Characterization of a 56–kb plasmid of Erwinia amylovora Ea322: its non–involvement in pathogenicity. Plasmid 24:12–24
Venisse JS, Barny MA, Paulin JP, Brisset MN (2003) Involvement of three pathogenicity factors of Erwinia amylovora in the oxidative stress associated with compatible interaction in pear. FEBS Lett 537:198–202
Venisse JS, Gullner G, Brisset MN (2001) Evidence for the involvement of an oxidative stress in the initiation of infection of pear by Erwinia amylovora. Plant Physiol 125:2164–2172
Wang D, Korban S, Pusey PL, Zhao Y (2012) AmyR is a novel negative regulator of Amylovoran production in Erwinia amylovora. PLoS One. https://doi.org/10.1371/journalpone0045038
Wei ZM, Beer SV (1996) Harpin from Erwinia amylovora induces plant resistance. Acta Hortic 411:223–226
Wei ZM, Laby RJ, Zumoff CH, Bauer DW, He SY, Collmer A, Beer SV (1992) Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science 257:85–88
Xie Z, Chen Z (2000) Harpin induced hypersensitive cell death is associated with altered mitochondrial functions in tobacco cells. Mol Plant-Microbe Interact 13:183–190
Yabuta Y, Mieda T, Rapolu M, Nakamura A, Motoki T, Maruta T, Yoshimura K, Ishikawa T, Shigeoka S (2007) Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. J Exp Bot 58:2661–2671
Zhao YF, Qi M (2011) Comparative genomics of Erwinia amylovora and related Erwinia species–what do we learn? Genes 2:627–639
Zhao YF, Blumer SE, Sundin GW (2005) Identification of Erwinia amylovora genes induced during infection of immature pear tissue. J Bacteriol 187:8088–8103
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors certify that 1) do not have any actual or potential conflict of interest, 2) the study described is original and has not been published previously, and is not under consideration for publication elsewhere, 3) all prevailing local, national and international regulations and conventions, and normal scientific ethical practices, have been respected.
Human participants and/or animals
No specific permits were required for the described studies.
Informed consent
All the authors certify that the work carried out in this research followed the principles of ethical and professional conduct. The study was designed by all authors for scientific research only, as well as data collection and analysis, decision to publish, or preparation of the manuscript. The author’s institutions were informed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Taheri Shahrestani, A., Abdollahi, H., Yakhchali, B. et al. Determination of the role of HrpN effector protein, as a key factor in course of interaction between Erwinia amylovora with chloroplasts of pear (Pyrus communis L.). J Plant Pathol 102, 1041–1050 (2020). https://doi.org/10.1007/s42161-020-00640-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-020-00640-0