Abstract
Purpose
To investigate how nodule size, nodule density, scan dose, slice thickness and reconstruction methods affect the performance of a deep learning (DL) model for detection of pulmonary nodules in phantom CT scans.
Materials and methods
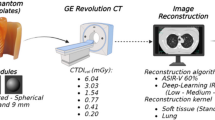
Spherical lung nodule phantoms of two different densities (− 630 HU and + 100 HU) and five different sizes (3, 5, 8, 10, and 12 mm) were inserted into an anthropomorphic chest phantom. CT data were scanned and reconstructed using three different tube current (10, 50, 200 mAs), two different slice thickness of 1 and 2 mm, four reconstruction methods (FBP-standard (FBP-STD), FBP-Y sharp kernel (FBP-YA), iDose4-standard kernel (iDose4-STD) and iDose4-Y sharp kernel (iDose4-YA). Evaluation of deep learning model focused on detection sensitivity and precision.
Results
According to the statistical results from the study, we found that the sensitivity and precision performance depends on the nodule sizes, nodule type, tube current, reconstruction methods and image thickness. Comparing the solid (100 HU) and ground-glass (GGO, − 630 HU) nodule phantoms, solid nodule phantom predictions are rarely affected by tube current, reconstruction methods and nodule sizes. Both sensitivity and precision are close to 100% in all solid nodule phantom prediction cases. While the sensitivity and precision metrics of GGO nodule phantoms change in a wide range from 42.9 to 100%. Larger nodule size and higher tube current gives a better sensitivity and precision for GGO nodule phantoms in most cases. We also analyze the relationships between the image thickness and the reconstruction methods. For 1-mm thickness images, iDose4-STD and FBP-STD shows a better result in both sensitivity and precision metrics. As for 2-mm thickness images, iDose-YA and FBP-YA gain a better performance.
Conclusion
The results of this phantom study demonstrated that high stability and flexibility of deep learning model can be used in daily clinical and screening practice.






Similar content being viewed by others
References
Naidich DP, Rusinek H, McGuinness G, et al. Variables affecting pulmonary nodule detection with computed tomography: evaluation with three-dimensional computer simulation. J Thorac Imaging. 1993;8:291–9.
Del CA, Paola F, Andrea C, et al. Missed lung cancer: when, where, and why? Diagn Interv Radiol. 2017;23(2):118–26.
Tang W, Wang JW, Wu N, et al. Computer-aided detection of nodule in low-dose CT screening for lung cancer. Chin J Radiol. 2012;46(7):619–23.
Awai K, Murao K, Ozawa A, et al. Pulmonary nodules at chest CT: effect of computer-aided diagnosis on radiologists’ detection performance. Radiology. 2004;230:347–52.
Beigelman-Aubry C, Raffy P, Yang W, et al. Computer-aided detection of solid lung nodules on follow-up MDCT screening: evaluation of detection, tracking, and reading time. AJR Am J Roentgenol. 2007;189:948–55.
Jeon KN, Goo JM, Lee CH, et al. Computer-aided nodule detection and volumetry to reduce variability between radiologists in the interpretation of lung nodules at low-dose screening computed tomography. Invest Radiol. 2012;47:457–61.
Sahiner B, Chan HP, Hadjiiski LM, et al. Effect of CAD on radiologists’ detection of lung nodules on thoracic CT scans: analysis of an observer performance study by nodule size. Acad Radiol. 2009;16:1518–30.
Cai JL, Xu DM, Liu SY, Cham MD. The added value of computer-aided detection of small pulmonary nodules and missed lung cancers. J Thorac Imaging. 2018;33:390–5.
Shen W, Zhou M, Yang F, Yang C, Tian J. Multi-scale convolutional neural networks for lung nodule classification. Inf Process Med Imaging. 2015;24:588–99.
Ciompi F, Chung K, Van Riel SJ, et al. Towards automatic pulmonary nodule management in lung cancer screening with deep learning. Sci Rep. 2017;7:464–79.
Causey JL, Zhang J, Ma S, et al. Highly accurate model for prediction of lung nodule malignancy with CT scans. Sci Rep. 2018;8(1):9286.
Liu K, Li Q, Ma JC, et al. Evaluating a fully automated pulmonary nodule detection approach and its impact on radiologist performance. Radiol Artif Intell. 2019;1(3):e180084.
Ronneberger O, Fischer P, Brox T. U-Net: convolutional networks for biomedical image segmentation. In: Navab N, Hornegger J, Wells W, Frangi A, editors. Medical image computing and computer-assisted intervention – MICCAI 2015, Lecture notes in computer science, vol. 9351. Springer, Cham (2015)
He, K, Zhang, X, Ren, et al. Deep residual learning for image recognition. In: 2016 IEEE conference on computer vision and pattern recognition (CVPR) 2016, 770–778.
Funama Y, Awai KD, Oda S, et al. Detection of nodules showing ground-glass opacity in the lungs at low-dose multidetector computed tomography: phantom and clinical study. J Comput Assist Tomogr. 2009;33(1):49–53.
Ohno Y, Yaguchi A, Okazaki T, et al. Comparative evaluation of newly developed model-based and commercially available hybrid-type iterative reconstruction methods and filter back projection method in terms of accuracy of computer-aided volumetry (CADv) for low-dose CT protocols in phantom study. Eur J Radiol. 2016;85:1375–82.
McNitt-Gray MF. AAPM/RSNA physics tutorial for residents: topics in CT-radiation dose in CT. Radiographics. 2002;22(6):1541–53.
Wielpütz Mark O, Wroblewski J, et al. Computer-aided detection of artificial pulmonary nodules using an ex vivo lung phantom: influence of exposure parameters and iterative reconstruction. Eur J Radiol. 2015;84(5):1005–11.
Suzuki K. A review of computer-aided diagnosis in thoracic and colonic imaging. Quant Imaging Med Surg. 2012;2:163–76.
Funding
The funding has been received from Shanghai Technology Committee Research Program (Grant no. 17411952400), Shanghai Hygiene Committee Intelligence Medical Research Program (Grant no. 2018ZHYL0101), Youth Medical Talents-Medical Imaging Practitioner Program (Grant no. 201972) and Shanghai Municipal Commission of Health and Family planning Program (Grant no. 20184Y0037).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Q., Li, Qc., Cao, Rt. et al. Detectability of pulmonary nodules by deep learning: results from a phantom study. Chin J Acad Radiol 2, 1–12 (2019). https://doi.org/10.1007/s42058-019-00015-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42058-019-00015-0