Abstract
Background
Resistant hypertension (rHTN) is defined as blood pressure (BP) of ≥ 140/90 mmHg despite treatment with at least three antihypertensive medications, including a diuretic. Endovascular ultrasound renal denervation (uRDN) aims to control BP alongside conventional BP treatment with antihypertensive medication. This analysis assesses the cost effectiveness of the addition of the Paradise uRDN System compared with standard of care alone in patients with rHTN from the perspective of the United Kingdom (UK) health care system.
Methods
Using RADIANCE-HTN TRIO trial data, we developed a state-transition model. Baseline risk was calculated using Framingham and Prospective Cardiovascular Münster (PROCAM) risk equations to estimate the long-term cardiovascular risks in patients treated with the Paradise uRDN System, based on the observed systolic BP (SBP) reduction following uRDN. Relative risks sourced from a meta-analysis of randomised controlled trials were then used to project cardiovascular events in patients with baseline SBP (‘control’ patients); utility and mortality inputs and costs were derived from UK data. Costs and outcomes were discounted at 3.5% per annum. Modelled outcomes were validated against trial meta-analyses and the QRISK3 algorithm and real-world evidence of RDN effectiveness. One-way and probabilistic sensitivity analyses were conducted to assess the uncertainty surrounding the model inputs and sensitivity of the model results to changes in parameter inputs. Results were reported as incremental cost-effectiveness ratios (ICERs).
Results
A mean reduction in office SBP of 8.5 mmHg with uRDN resulted in an average improvement in both absolute life-years (LYs) and quality-adjusted life-years (QALYs) gained compared with standard of care alone (0.73 LYs and 0.67 QALYs). The overall base-case ICER with uRDN was estimated at £5600 (€6500) per QALY gained (95% confidence interval £5463–£5739 [€6341–€6661]); modelling demonstrated > 99% probability that the ICER is below the £20,000–£30,000 (€23,214–€34,821) per QALYs gained willingness-to-pay threshold in the UK. Results were consistent across sensitivity analyses and validation checks.
Conclusions
Endovascular ultrasound RDN with the Paradise system offers patients with rHTN, clinicians, and healthcare systems a cost-effective treatment option alongside antihypertensive medication.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Our analysis, based on the RADIANCE-HTN TRIO trial data, suggests that endovascular ultrasound renal denervation (uRDN) is likely to be a cost-effective option for patients with resistant hypertension (rHTN). |
Treating rHTN with the addition of endovascular uRDN instead of standard-of-care antihypertensive medications alone leads to long-term gains in life-years and quality-adjusted life-years. |
The results are robust and show that the cost effectiveness of endovascular uRDN is most sensitive to the level of relative risk of stroke with reduction in systolic blood pressure with uRDN and health utility associated with a stroke. |
1 Introduction
Uncontrolled hypertension leads to higher risk of cardiovascular complications and mortality, resulting in a twofold increase in cardiovascular morbidity and mortality compared with patients responsive to treatment [1,2,3,4]. Among uncontrolled patients, resistant hypertension (rHTN) is defined as an office systolic blood pressure (SBP) of ≥ 140 mmHg and/or a diastolic blood pressure (DBP) of ≥ 90 mmHg, despite the use of at least three appropriately administered antihypertensive medications, including a diuretic [5, 6]. rHTN is a clinically important problem affecting 12–15% of the treated hypertensive population [7].
Patients with rHTN have a substantial unmet need for a safe and durable treatment that does not add to the daily burden of adherence to multiple medications and provides significant clinical benefit without poorly tolerated adverse effects. The Paradise endovascular ultrasound renal denervation (uRDN) reduces BP alongside conventional antihypertensive treatment by delivering ultrasound energy to thermally ablate the renal sympathetic nerves that play an important role in the pathophysiology of rHTN [8]. The recently published RADIANCE-HTN TRIO multicentre, randomised, sham-controlled trial of Paradise uRDN in patients with rHTN reported a median SBP reduction at 2 months follow-up of 8.0 mmHg (interquartile range [IQR] − 16.4 to 0.0) compared with baseline measurements in the intention-to-treat population for the primary endpoint of daytime ambulatory SBP [9]. When using office-based measurements, a mean SBP reduction of 8.5 ± 19.1 mmHg was reported in the intention-to-treat population at 2 months [9]. Reductions of this magnitude in antihypertensive drug trials have been shown to be clinically relevant, leading to a 15–20% reduction in major cardiovascular events [10], and model-based projections of major cardiovascular event reductions suggest a reduction of 26% in relative risk and 2.9% in absolute risk [11].
In addition to evidence of clinical efficacy, safety, and clinical effectiveness, healthcare payers increasingly require cost-effectiveness analyses to judge the value of health technologies. Previous studies based on results from the SYMPLICITY HTN-2 and DENERHTN trials demonstrated the cost effectiveness of renal denervation for rHTN [10, 12, 13]. However, there is a need for updated economic modelling based on the results from contemporary trials utilising newer RDN technologies and rigorous trial designs. The sham-controlled RADIANCE-HTN TRIO trial was conducted incorporating several trial design features addressing the limitations of prior rHTN trials [9, 14].
Using SBP reduction data from RADIANCE-HTN TRIO, we sought to develop a decision-analytic model to predict long-term cardiovascular consequences and to address the research question of whether the addition of endovascular uRDN to standard of care (SoC) compared with SoC alone is a cost-effective option in the long-term for patients with rHTN. As a sensitivity analysis, we also modelled the cost effectiveness of renal denervation outside of rigorously controlled clinical studies using real-world data analysis of the long-term outcomes from the ACHIEVE study [15].
2 Methods
2.1 Study Design
This economic evaluation was undertaken from the perspective of the United Kingdom (UK) health care system, as this is a key reference country for the use of cost-effectiveness analyses. The analysis covers direct health and social care costs and uses data from the RADIANCE-HTN TRIO trial [9], which had Ethics Committee and Institutional Review Board approvals from each site participating in the study. The evaluation is reported in accordance with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement and the UK National Institute for Health and Care Excellence (NICE) reference methods [16, 17]. A state-transition (Markov) model was used to project the impact of treatment with the Paradise uRDN system (ReCor Medical Inc., Palo Alto, CA, USA) plus SoC compared with SoC alone. A lifetime time horizon was used to capture all potential cost and outcome effects of the intervention, and both were discounted at 3.5% per annum [17].
2.2 Patient Population
In the base-case analysis, the rHTN population considered in the model was based on RADIANCE-HTN TRIO trial inclusion and exclusion criteria [9]. The trial was conducted across 28 tertiary centres in the United States and Europe, and included patients aged 18–75 years with office BP ≥ 140/90 mmHg despite three or more antihypertensive medications, including a diuretic. Eligible patients were switched to a once-daily, fixed-dose, single-pill combination of a calcium channel blocker, an angiotensin receptor blocker, and a thiazide diuretic. After 4 weeks of standardised therapy, 136 patients with daytime ambulatory BP of at least 135/85 mmHg were randomly assigned (1:1) to uRDN (n = 69) or a sham procedure (n = 67).
2.3 Model Structure
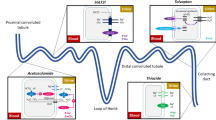
The model had a 1-month cycle length with half-cycle correction incorporated. The model included 11 mutually exclusive health states to represent disease progression. This structure was based on previous economic evaluations and uses SBP as a surrogate endpoint to predict cardiovascular and renal disease risks (Fig. 1) [10, 12]. The model projected six clinical events: angina pectoris/coronary heart disease (AP/CHD), end-stage renal disease (ESRD), myocardial infarction (MI), heart failure (HF), stroke, and all-cause mortality. All patients start in the hypertension health state and move to a different health state (with different health-related quality of life [HRQoL] and costs) when an event occurs. Death is an absorbing health state and can occur at any time. Consistent with previous cost-effectiveness models, we used risk equations based on Framingham and the Prospective Cardiovascular Münster (PROCAM) study to model how patients transition through the different health states [18].
Structure of the cost-effectiveness model. *Death is an absorbing health state that can be entered at any given time. #Memory has been incorporated to track ESRD status throughout the model time horizon. ^ Memory has been incorporated to track heart failure status in stroke patients. AP angina pectoris, CHD coronary heart disease, ESRD end-stage renal disease, MI myocardial infarction
Compared with previous modelling, three major modifications were made to more effectively capture the true clinical impact of SBP changes following renal denervation. First, and most importantly, we changed the approach to modelling the effect of SBP reduction. Risk equations used in previous models to predict the downstream effect of a change in SBP on long-term cardiovascular risk were based on epidemiological observational data (e.g., Framingham and PROCAM) and therefore do not accurately reflect the change in risk of clinical events resulting from a change in SBP due to an intervention to actively reduce blood pressure. To address this, we translated the SBP reduction associated with uRDN to a reduction of clinical events based on the relative risks reported by the meta-analysis of Thomopoulos et al. in 2014 (55 randomised controlled trials [RCTs] of antihypertensive medication in 195,267 individuals) [19]. In contrast to the meta-analyses of Rahimi et al. [20] and Ettehad et al. [21], Thomopoulos et al. included only RCTs with antihypertensive treatment intent. Second, we added a recurrent stroke health state to capture the significantly elevated long-term risks of a stroke and the reduced HRQoL for patients with recurrent stroke [22, 23]. Third, we incorporated ‘memory’ functionality for ESRD. The model structure already incorporated a memory to track HF status in stroke patients, as this significantly impacts HRQoL. Supplementing with a ‘memory’ for ESRD captures the HRQoL-lowering effects and continued high costs of ESRD in subsequent events, e.g., an MI or stroke.
2.4 Clinical and Health-Related Quality-of-Life Inputs
Table 1 summarises the key parameters used in the model. A full description is provided in the e-Appendix (see the electronic supplementary material). The model used office-based SBP from the RADIANCE-HTN TRIO trial since current cardiovascular risk equations [18, 24] and published meta-analyses of the clinical effect of changes in SBP [19,20,21] are calibrated using office SBP measurements. The baseline office SBP across both arms of the trial was 155.3 mmHg, with a mean reduction of 8.5 ± 19.1 mmHg in the uRDN arm at 2 months. No sham intervention would be performed in real-world clinical practice and any placebo effect would be part of the overall treatment effect observed for the intervention. Therefore, the base-case analysis assumes that no SBP reduction was associated with SoC alone (continued medical management for rHTN). Similar assumptions have been used and were accepted previously for health technology assessments in the UK [25, 26]. Other model clinical parameters were derived from literature searches and previously published models. Utilities for specific health states were drawn from a variety of sources, including previous clinical trials and economic evaluations of cardiovascular interventions and HRQoL studies (see Table 1). Further details of the sources of utilities are provided in eAppendix 1.3 Tables 3 and 4.
2.5 Cost Inputs
Costs used in the model are also outlined in Table 1 and e-Appendix 1. Costs were taken from published sources, including the Personal Social Services Research Unit (PSSRU) Pay and Prices Index and UK Department of Health and Social Care drugs and pharmaceutical electronic Market Information Tool (eMIT) [27, 28]. The cost of the uRDN procedure is estimated to be £6500 (€7545) [costs provided by the manufacturer], including both the costs for the catheter and the hospital treatment costs. When required, costs were inflated to 2021/2022 GBP/£ levels using NHS cost inflation indices [27], and results were converted to Euros using the conversion rate by the European Central Bank as of 8 August 2023 (£1.0000 = €1.1607).
2.6 Data Analysis
Results were reported as incremental cost-effectiveness ratios (ICERs). This was done by calculating the ratio of the difference in mean costs and mean change in quality-adjusted life-years (QALYs) and life-years (LYs) between Paradise uRDN plus SoC and SoC alone. To provide full insight into the robustness of the results, a 95% confidence interval (CI) around the ICER has been calculated. The box method was applied as a simplified method to calculate this interval to avoid additional complexity [29].
One-way sensitivity analysis (OWSA) and probabilistic sensitivity analysis (PSA) were conducted to assess the uncertainty surrounding the model inputs and sensitivity of the model results to changes in parameter inputs. OWSA was performed using realistic minimum and maximum individual model inputs (one at a time); for all model parameters, the minimum and maximum plausible values for univariate analysis were defined as the lower and upper 95% confidence limits (95% CIs). For the PSA, all parameters were varied simultaneously and results were recorded for 1000 iterations, which was enough to provide stable results. Most variables were assumed to have a normal distribution, except for proportions, probabilities, and utility estimates, which were all varied using a beta distribution. Hazard ratios were varied using a gamma distribution. An overview of which parameters were included in each analysis is provided in the e-Appendix.
Several scenario analyses were used to explore the impact of the model’s structural assumptions. For insight into the real-world cost effectiveness of uRDN, we used the 12-month results of the ACHIEVE study, which included patients treated with the uRDN system (n = 96) [15]. The ACHIEVE study observed the effectiveness of the uRDN system in reducing BP, demonstrating a 15.0 mmHg reduction in mean office SBP. The mean baseline office SBP in ACHIEVE was 176 versus 155.3 mmHg in the RADIANCE-HTN TRIO trial [9, 15]. A scenario was also included that uses the estimate of 5 mmHg SBP reduction to test the cost-effectiveness, in case data from placebo-subtracted sham-controlled trials were used.
A patient-level simulation component explored the impact of modelling a heterogeneous patient population, which can cause biased results when there is a non-linear relationship between risk factors and cardiovascular event risks (Jensen’s inequality) [30]. The simulation model uses random sampling to create a virtual patient cohort based on defined patient characteristics and the correlation between them, as found in the RADIANCE-HTN TRIO trial (e-Appendix 2). Each patient from the cohort is then run through the model’s existing Markov structure. The results are averaged to achieve an overall cohort result to compare with the base-case deterministic results. Patient-level simulation represents a novel approach in hypertension modelling that was not featured in previously published models [9, 10].
For external validation, modelled relative risks and hazard ratios were compared with those presented in meta-analyses conducted by Rahimi et al. [20] and Ettehad et al. [21]. Absolute risks were compared with clinical examples of the QRISK3 algorithm, presented by Hippisley-Cox et al. [31].
3 Results
3.1 Base-Case Results
The base-case analysis indicates uRDN plus SoC results in a mean improvement in LYs and QALYs per patient compared with SoC alone (15.14 vs. 14.37 Lys, and 12.12 vs. 11.49 QALYs) over a lifetime horizon. Higher mean costs are associated with uRDN plus SoC compared with SoC alone (£34,784 vs. £31,261 per patient [€40,374 vs. €36,284]). With mean incremental QALYs of 0.629 at £3523 (€4090) incremental costs, the overall cost per QALY gained is estimated to be £5600 (95% CI £5463–£5739) [€6500; €6341–€6661] (see Table 2). This ICER falls well below the UK NICE willingness-to-pay (WTP) threshold of £20,000–£30,000 (€23,214–€34,821) per QALY [15]. The breakdown of model results around specific downstream event rates and averted events, as well as costs associated with individual events, are provided in Table 3.
3.2 Uncertainty and Scenario Analyses
The OWSA results indicate that the model’s findings are relatively insensitive to uncertainty around individual parameter estimates. Figure 2 presents a tornado diagram of the most influential parameters, which include several of the relative risks applied to the intervention arm baseline cardiovascular risks as well as the utility of stroke. The PSA, based on 1000 iterations (see Fig. 3), shows there is > 99% probability of the uRDN system being cost effective at a £30,000 (€34,821) and £20,000 (€23,214) WTP threshold.
Scenario 1, where relative risks from the meta-analysis by Ettehad et al. were applied, results in an ICER of £10,554 (€12,250). Scenario 2, with hazard ratios by Rahimi et al., resulted in an ICER of £13,616 (€15,804) (Table 4). The model results are also robust when making alternative assumptions around the model’s structure (Scenarios 3–5), with ICERs ranging from £5342 to £5624 (€6201 to €6527). When the inputs observed in the real-world ACHIEVE study were inputted to the model (Scenario 6), a similar result was found, resulting in an ICER of £371 (€431). Results of the patient-level simulation (Scenario 7) show consistency with the base-case deterministic results, with an ICER of £6087 (€7065) and placebo-subtracted SBP reduction of − 5 mmHg (Scenario 8) resulting in an ICER of £12,853 (€14,919). Detailed results are presented in e-Appendix 2.
3.3 Validation
While the base-case ICER of the current model was higher than reported in the previously published models, this difference reflects the difference in effect size measured in the RADIANCE-HTN TRIO trial compared with the SYMPLICITY HTN-2 trial published many years ago and investigating a more severe hypertensive population/cohort. External validation indicates the model results are concordant with absolute risk estimates as per QRISK3® as well as with relative risk estimates from Thomopoulos et al. and Rahimi et al. [19, 20]. Detailed validation results are presented in e-Appendix 3.
4 Discussion
Our results show that addition of uRDN to SoC is a cost-effective treatment strategy for patients with rHTN, with an ICER of £5600 (€6500), provided that the effects of uRDN as observed in the RADIANCE-HTN TRIO trial are shown to be durable and safe with longer-term follow-up [9]. Modelling demonstrated a > 99% probability that this is cost effective in the UK based on a WTP threshold of £20,000 (€23,214) [17]. This conclusion was robust to our various sensitivity and scenario analyses that produced ICERs that all remained below this threshold. Model validity was verified against previous economic models [10, 12, 13].
Previously published cost-effectiveness analyses have shown the Symplicity radiofrequency renal denervation system to be a cost-effective use of resources [10, 12, 32]. An example is the trial-based French economic analysis, undertaken alongside the DENERHTN clinical trial [32]. This study modelled a 6-month time horizon and estimates the cost per mmHg reduction in daytime ambulatory SBP. The study uses an SBP reduction of 5.9 mmHg with radiofrequency RDN and explores the use of relative risks derived from a meta-analysis. However, in contrast to the present study, these previous economic evaluations have not been based on latest generation renal denervation trial data of uRDN.
Our model included several significant updates compared with previously published decision analytic models of cost effectiveness of renal denervation. Knowledge and understanding of the role of cardiovascular risk factors has increased over the last decade since previous models were published, and there are now several meta-analyses of long-term data available that investigate the effect of actively lowering SBP on cardiovascular outcomes [19,20,21]. These analyses move from examining the association of SBP with the occurrence of cardiovascular events to the association of reduction in SBP to changes in event rates, reflecting more faithfully the likely effects of clinical interventions. Given that the meta-analyses are based on multiple interventional studies, while risk equations are based on epidemiological data, the former are more appropriate for modelling the effect of an intervention on long-term clinical outcomes. As a result, we drew upon the use of the meta-analysis by Thomopoulos et al. [19] for the base-case analysis rather than calculating risks in the intervention arm directly using risk equations, as has been done in previous cost-effectiveness models [9, 10, 24]. That these data are more clinically relevant to assess the effects of a therapeutic intervention for rHTN is discussed in a recent editorial from Böhm and Lauder [33]. Other enhancements in the model’s structure included the addition of a health state for a recurrent stroke as well as the addition of ‘memory function’ to better capture the impact of ESRD through a lifetime. While these two modifications had a lesser impact on the results versus previous renal denervation economic models, we believe that they contribute to making the model more reflective of real-world clinical practice.
The base case for this model used the 8.5 mmHg reduction in mean office SBP observed in the RADIANCE-HTN TRIO study after 2 months [9]. However, the results from the ACHIEVE study show a larger intervention effect that is related to the higher baseline BP [34]. Of note, the BP-lowering effect is maintained after 1 year in the ACHIEVE trial, and there is now evidence of durability with catheter based RDN through to 9 years [35, 36]. The expected use of uRDN in clinical practice could be in patients more similar to published real-world clinical studies and individuals presenting at screening for the RADIANCE-HTN TRIO cohort than at baseline. In which case, the real-world outcomes scenarios might be closer to clinical practice than the base-case analysis presented here [8]. Nonetheless, we used these in sensitivity analyses, selecting the more conservative RADIANCE-HTN TRIO findings for our base case. Given that the technology assessed is a medical device, it can be relevant to consider the impact of operator skills on treatment effect and applicability of outcome results of trial results. However, in the case of RDN, there is no evidence to indicate an impact of operator experience. Prior uRDN experience was not an inclusion criteria of the RADIANCE-HTN study programme (including the TRIO [8] and SOLO [37] trials) and was applicable to a small fraction of proceduralists included in these trials. The consensus statement of the European Society of Cardiology Council on Hypertension and Association of Percutaneous Cardiovascular Interventions recommend RDN should be overseen by a multidisciplinary team and should include experts in hypertension and percutaneous cardiovascular interventions [38]. There are some potential limitations to this analysis. First, daytime ambulatory BP was the primary outcome of the RADIANCE-HTN TRIO trial. Daytime ambulatory BP measurement is considered the standard method of BP measurement, providing the average of repeated automatic BP readings over a defined period, usually 24 h. They are usually lower than office BP readings, where the ‘white coat effect’ can result in higher BP readings [9]. However, all validated risk equations estimating the relationships between BP and long-term clinical effects used in the current study are based on office-based BP measurements, which has historically been the BP endpoint captured in clinical trials [19,20,21]. As a result, we used the office BP results for this analysis.
Second, measuring the unbiased impact of renal denervation on BP in patients with rHTN can be challenging as it depends not only on the procedure but also on the effect of potential changes in concomitant antihypertensive medication over time. This is another reason why we chose to use data from RADIANCE-HTN TRIO to model treatment effects; the study design optimised the ability to hold background medications constant. Nonetheless, we must recognise that changes in medication adherence over the course of clinical studies may affect these estimates.
Third, HRQoL utility values used in the economic model came from a range of sources and were therefore based on different collection methods (e.g., EQ-5D vs. time trade-off) and included non-UK population sources.
Fourth, our model assumed no effect on SBP for patients receiving SoC only. In clinical practice, patients would remain on treatment as they are, or alternative therapies could be tested. We did not undertake an evaluation of alternative therapies such as other pharmaceutical options (e.g., spironolactone) or other device-based treatments for hypertension. However, patients included in RADIANCE-HTN TRIO were considered to be resistant to pharmaceutical treatment, having previously attempted and exhausted multiple drug options, and as such, in routine clinical practice, no sham procedure would be performed. We therefore felt that it was not appropriate to consider the sham arm of the RADIANCE-HTN TRIO trial as equivalent to clinical SoC [39]. This issue has been previously discussed and accepted by NICE in the UK for two health technology appraisals considering data from sham-controlled trials [25, 26].
Fifth, the meta-analyses used in this study to translate the BP-lowering effect of uRDN into a reduction in long-term cardiovascular complications are based on RCT data from a basket of antihypertensive drug interventions [19,20,21]. The question therefore remains as to whether the treatment effect on SBP is transferable outside this treatment class and to other BP-reducing approaches, including uRDN.
Finally, as with previous economic models, long-term treatment effect assumptions must be made concerning the durability of the therapeutic effect of uRDN. Supported by recent data showing a reduction in SBP with RDN out to 9 years follow-up, our model-based analysis assumes no waning of treatment effect [36]. The base-case analysis of this model is based on the intervention arm of a tightly controlled sham-controlled trial. In general, tightly monitored sham-controlled trials of renal denervation have shown more modest BP reductions as compared with real-world registries such as the ACHIEVE study [15]. Estimates of treatment effect sizes of renal denervation in rHTN patients range from as high as 20 mmHg in real-world registries to as low as 5 mmHg in placebo-subtracted, sham-controlled trials, which may be less standardised to real-world practice where sham procedures are not offered. Using this range of effect sizes, scenario analyses confirm uRDN would be cost effective at the accepted threshold of £20,000 per QALY.
5 Conclusions
Endovascular ultrasound RDN with the Paradise System in addition to SoC offers patients, clinicians, and healthcare systems a cost-effective alternative to traditional antihypertensive drug therapy alone in resistant HTN. This conclusion was robust to our various sensitivity and scenario analyses, which all produced ICERs below the WTP threshold of £20,000 per QALY from NICE in UK. While our analysis shows uRDN to be an important addition to the treatment armamentarium for resistant HTN, the scale of uncontrolled HTN in the population requires the continued need for optimisation of lifestyle and pharmaceutical interventions [40].
Change history
08 May 2024
A Correction to this paper has been published: https://doi.org/10.1007/s41669-024-00485-8
References
Borghi C, Tubach F, De Backer G, et al. Lack of control of hypertension in primary cardiovascular disease prevention in Europe: results from the EURIKA study. Int J Cardiol. 2016;218:83–8.
Diaz KM, Booth JN 3rd, Calhoun DA, et al. Healthy lifestyle factors and risk of cardiovascular events and mortality in treatment-resistant hypertension: the reasons for geographic and racial differences in stroke study. Hypertension. 2014;64:465–71.
Foreman KJ, Marquez N, Dolgert A, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet. 2018;392:2052–90.
World Health Organization. A global brief on hypertension—silent killer, global public health crisis: World Health Day 2013. World Health Organization; 2013. https://apps.who.int/iris/handle/10665/79059.
Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53–90.
National Institute for Health Care and Excellence. Hypertension in adults: diagnosis and management [NG136]; 2019. https://www.nice.org.uk/guidance/ng136.
Judd E, Calhoun DA. Apparent and true resistant hypertension: definition, prevalence and outcomes. J Hum Hypertens. 2014;28:463–8.
ReCor Medical. The Paradise™ Renal Denervation System; 2021. https://www.recormedical.com/our-technology/.
Azizi M, Sanghvi K, Saxena M, et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet. 2021;397:2476–86.
Geisler BP, Egan BM, Cohen JT, et al. Cost-effectiveness and clinical effectiveness of catheter-based renal denervation for resistant hypertension. J Am Coll Cardiol. 2012;60:1271–7.
Schmieder RE, Mahfoud F, Mancia G, et al. Clinical event reductions in high-risk patients after renal denervation projected from the global SYMPLICITY registry. Eur Heart J Qual Care Clin Outcomes. 2023;9:575–82.
Gladwell D, Henry T, Cook M, Akehurst R. Cost effectiveness of renal denervation therapy for the treatment of resistant hypertension in the UK. Appl Health Econ Health Policy. 2014;12:611–22.
Bulsei J, Darlington M, Durand-Zaleski I, Azizi M, DENERHTN Study Group. How to perform a cost-effectiveness analysis with surrogate endpoint: renal denervation in patients with resistant hypertension (DENERHTN) trial as an example. Blood Press. 2018;27:66–72.
Kandzari DE, Mahfoud F, Weber MA, et al. Clinical trial design principles and outcomes definitions for device-based therapies for hypertension: a consensus document from the Hypertension Academic Research Consortium. Circulation. 2022;145:847–63.
Daemen J, Mahfoud F, Kuck KH, et al. Safety and efficacy of endovascular ultrasound renal denervation in resistant hypertension: 12-month results from the ACHIEVE study. J Hypertens. 2019;37:1906–12.
Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Value Health. 2013;16:e1–5.
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal; 2013. https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781.
Voss R, Cullen P, Schulte H, Assmann G. Prediction of risk of coronary events in middle-aged men in the Prospective Cardiovascular Münster Study (PROCAM) using neural networks. Int J Epidemiol. 2002;31:1253–62.
Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension. 1. Overview, meta-analyses, and meta-regression analyses of randomized trials. J Hypertens. 2014;32:2285–95.
Rahimi K, Bidel Z, Nazarzadeh M, et al. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet. 2021;397:1625–36.
Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–67.
Edwards JD, Kapral MK, Fang J, Swartz RH. Long-term morbidity and mortality in patients without early complications after stroke or transient ischemic attack. Can Med Assoc J. 2017;189:e954–61.
Wang YL, Pan YS, Zhao XQ, et al. Recurrent stroke was associated with poor quality of life in patients with transient ischemic attack or minor stroke: finding from the CHANCE trial. CNS Neurosci Ther. 2014;20:1029–35.
Framingham Heart Study. Epidemiological Background and Design: The Framingham Heart Study; 2020. https://framinghamheartstudy.org/fhs-about/history/epidemiological-background/.
National Institute for Health and Care Excellence. TA260: botulinum toxin type A for the prevention of headaches in adults with chronic migraine; 2012. https://www.nice.org.uk/guidance/ta260.
National Institute for Health and Care Excellence. TA349: dexamethasone intravitreal implant for treating diabetic macular oedema; 2015. https://www.nice.org.uk/guidance/ta349.
Jones KC, Weatherly H, Birch S, Castelli A, Chalkley M, Dargan A, et al. Aunit Costs of Health and Social Care 2022 Manual. Technical report. Personal Social Services Research Unit (University of Kent) and Centre for Health Economics (University of York), Kent; 2023. https://kar.kent.ac.uk/100519/.
Department of Health and Social Care. Drugs and pharmaceutical electronic market information tool (eMIT); 2021. https://www.gov.uk/government/publications/drugs-and-pharmaceutical-electronic-market-information-emit.
Polsky D, Glick HA, Willke R, Schulman K. Confidence intervals for cost-effectiveness ratios: a comparison of four methods. Health Econ. 1997;6:243–52.
Denny M. The fallacy of the average: on the ubiquity, utility and continuing novelty of Jensen’s inequality. J Exp Biol. 2017;220:139–46.
Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. Br Med J. 2017;357: j2099.
Azizi M, Sapoval M, Gosse P, et al. Optimum and stepped care standardized antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet. 2015;385:1957–65.
Böhm M, Lauder L. Blood pressure and renal denervation with ultrasound: another step forward. Lancet. 2021;397:2441–3.
Messerli FH, Bangalore S, Schmieder RE. Wilder’s principle: pre-treatment value determines post-treatment response. Eur Heart J. 2015;36:576–9.
Sesa-Ashton G, Nolde JM, Muente I, et al. Catheter-based renal denervation: 9-year follow-up data on safety and blood pressure reduction in patients with resistant hypertension. Hypertension. 2023;80:811–9.
Zeijen VJM, Feyz L, Nannan Panday R, et al. Long-term follow-up of patients undergoing renal sympathetic denervation. Clin Res Cardiol. 2022;11:1256–68.
Azizi M, Schmieder RE, Mahfoud F, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391:2335–45.
Barbato E, Azizi M, Schmieder RE, et al. Renal denervation in the management of hypertension in adults. A clinical consensus statement of the ESC Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2023;44:1313–30.
Hawkins N, Scott DA. Cost-effectiveness analysis: discount the placebo at your peril. Med Decis Making. 2010;30:536–43.
Lauder L, Azizi M, Kirtane AJ, et al. Device-based therapies for arterial hypertension. Nat Rev Cardiol. 2020;17:614–28.
Khan SS, Ning H, Shah SJ, et al. 10-Year risk equations for incident heart failure in the general population. J Am Coll Cardiol. 2019;73:2388–97.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by ReCor Medical.
Conflict of interest
Rod S. Taylor has received personal consultancy fees from ReCor Medical. Anthony Bentley and Kaylie Metcalfe are employees of Mtech Access, contractor for ReCoR Medical. Melvin D. Lobo has received grant support from ReCor Medical and personal fees from ReCor Medical, Medtronic, CVRx, Ablative Solutions, Vascular Dynamics, ROX Medical and Tarilan Laser Technologies, as well as grants from Medtronic. Ajay J. Kirtane reports institutional funding to Columbia University and/or the Cardiovascular Research Foundation from Medtronic, Boston Scientific, Abbott Vascular, Abiomed, CSI, Siemens, Philips, ReCor Medical, and Neurotronic. In addition to research grants, institutional funding includes fees paid to Columbia University and/or the Cardiovascular Research Foundation for consulting and/or speaking engagements in which Ajay J. Kirtane controlled the content; personal: consulting from IMDS. Michel Azizi has received research grants from the French Ministry of Health, Quantum Genomics, and the European Horizon 2020 programme; has received grant support and nonfinancial support from ReCor Medical and Idorsia; and has received personal fees from CVRx. Christopher Clark has received personal fees from ReCor Medical and Bayer. Kieran Murphy is an employee of ReCor Medical. Jennifer H. Boer, Marjolijn van Keep and An Thu Ta were previously employed by BresMed, a contractor for ReCor Medical. Neil C. Barman is an employee of ReCor Medical. Garrett Schwab has received personal fees from ReCor Medical. Roland E. Schmieder has received research grants, paid to the institution, from ReCor Medical, Medtronic Inc. and Ablative Solutions, and has received personal consultancy and speaker fees from ReCor Medical, Medtronic Inc and Ablative Solutions.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication (from patients/participants)
Not applicable.
Code availability
The cost-effectiveness model was developed in Microsoft Excel 365 (Microsoft Corporation, Redmond, WA, USA). Any additional information about model programming is available from the corresponding author upon request.
Author contributions
JNB, MvK, and RA constructed the first version of the economic model, which was updated by AB and KM. RST and KM wrote the first draft of this manuscript. All authors revised and contributed to subsequent versions of the manuscript, and the final version was approved by all authors.
Additional information
The original online version of this article was revised: In this article Tab 2 and 3 have been updated.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Taylor, R.S., Bentley, A., Metcalfe, K. et al. Cost Effectiveness of Endovascular Ultrasound Renal Denervation in Patients with Resistant Hypertension. PharmacoEconomics Open (2024). https://doi.org/10.1007/s41669-024-00472-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s41669-024-00472-z