Abstract
Background
In 2015, Spain launched a national eradication strategy for hepatitis C virus (HCV), resulting in the highest treatment rate in Europe and substantial reductions in HCV prevalence. However, to achieve the goal of HCV elimination, it is necessary to scale-up the diagnosis, treatment, and management of HCV infection.
Objective
Our aim was to assess the prevalence, incidence, and cost effectiveness of scaling-up compared with status quo scenarios.
Methods
A compartmental dynamic transmission model was developed comprising of a cascade of care and a liver progression module. Cost and quality-of-life inputs were sourced from the literature. Key outcomes were the prevalence and incidence of HCV and the incremental cost per quality-adjusted life-year (QALY) and per life-year (LY). Outcomes for a hypothetical elimination strategy were compared with the status quo.
Results
The base-case analysis found that scaling-up testing and treatment reduced both the prevalence and incidence of HCV over time, resulting in incremental costs per QALY and LY of €13,291 and €12,285 respectively, compared with the status quo. The main drivers of the cost-effectiveness results included cost of diagnosis, cost of treatment, proportion of people who are unaware, percentage of population who inject drugs, and calibration parameters related to HCV infection prevalence.
Conclusions
This analysis demonstrated that scaling-up testing and treatment with direct-acting antivirals may be an efficient strategy for reducing the incidence and prevalence of HCV and may help achieve HCV elimination goals in Spain.
Similar content being viewed by others
Despite the progress made in Spain towards hepatitis C virus (HCV) elimination, further scaling-up of diagnosis, treatment and management is needed to achieve this goal. |
Our analysis found that such an increase in testing and treatment of HCV may be an efficient and cost-effective strategy to reduce incidence and prevalence. |
1 Introduction
The hepatitis C virus (HCV) is a predominantly bloodborne virus that causes both acute and chronic infection [1]. Most acute infections are asymptomatic and do not lead to life-threatening complications. While approximately one-third of infections clear within 6 months without any treatment, the remaining two-thirds develop chronic hepatitis C (CHC), which ranges in severity from mild to life-threatening disease and may lead to severe complications such as liver cirrhosis and cancer [1, 2].
CHC has a high prevalence, and recent data estimates the number of persons with chronic infection to be approximately 58 million globally, with around 400,000 people dying annually from CHC [3]. The prevalence of HCV across European countries varies between 0.2% and 5% of the population [4]. While the prevalence of HCV in Spain is currently estimated at around 0.22% of the population, representing approximately 120,000 people, Spain has one of the largest population of migrants, a group historically associated with higher prevalence of HCV [5, 6]. Although the number of cases has been declining, approximately two-thirds of infected individuals are unaware, and thus untreated, of their CHC [7].
Treatment for CHC includes direct-acting antivirals (DAAs), which have high efficacy (achievement of 95% sustained virological response [SVR]) and are well tolerated, with a short 12-week treatment duration [8]. Patients who successfully eliminate HCV through treatment experience significant improvements in health-related quality of life (HRQoL) by preventing complications due to advanced liver disease and reducing non-liver complications such as fatigue, irritability, depression, muscle pain, joint pain and cognitive impairment [9]. However, access to DAAs remains limited globally; in 2019, only 21% of individuals living with HCV were reported to be aware of their diagnosis and only two-thirds of them were being treated with DAAs in 2019 [2].
In 2016, the World Health Organization (WHO) adopted the goal of eliminating HCV as a public health problem by the year 2030. It set out to achieve this by targeting an 80% reduction in new CHC infections and a 65% reduction in mortality from 2015 levels [10]. In parallel, in 2015, Spain implemented its own national HCV strategy with the aim of reducing HCV-related morbidity and mortality [11]. This strategy included free universal access to DAA treatments, and, subsequently, Spain has made substantial progress towards elimination in recent years, but continuing on this trajectory will require taking additional steps, such as increased screening and improving link to care [12, 13].
Given the costs associated with managing the disease due to hepatic and extrahepatic manifestations, as well as the indirect costs incurred from impaired HRQoL and loss of work productivity, investing in testing and treatment may reduce the overall economic burden associated with HCV [14]. The objective of the current study was to quantify the clinical and economic benefits, from a societal perspective, of scaling-up HCV testing and treatment, versus the status quo, on the elimination of HCV infection in Spain.
2 Methods
2.1 Model Structure
A compartmental dynamic transmission model, from a societal perspective, was developed in Microsoft Excel® (with visual basic for applications) [Microsoft Corporation, Redmond, WA, USA]. After considering the research question and the aims of the analysis, the model structure was chosen for its ability to represent the typical HCV pathway. Dynamic transmission models are not as common in health economics analyses than structurally less complex models such as decision trees or Markov models, due to an added level of complexity and data requirements [15, 16]. However, in disease areas where treatment and exposure are dependent factors, a dynamic model is required to capture all relevant aspects of disease. For this reason, dynamic transmission models are more widely used in infectious diseases and the method was deemed appropriate to model HCV elimination in Spain [15, 16].
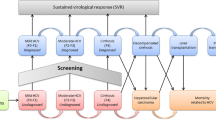
The model was conceptualized as having two main components, with the first being a cascade-of-care module (Fig. 1) and the second being a liver disease module (Fig. 2). All individuals entered the model as never infected; from this state, all individuals were susceptible of being infected at a rate of infection, which was a constant probability multiplied by the prevalence of infection. The liver disease compartment contained a module for those chronically infected and those cured (Fig. 2). Death was the absorbing state; chronically infected or cured individuals could transition to death from any of the health states. In order to align with the WHO target elimination goals, the model timeframe was from 2021 to 2030. Both costs and health effects were discounted at 3.0% per annum [17]. Compartment occupancy were calculated based on a set of differential equations that do not correspond to traditional fixed model cycles; however, costs and utilities were accrued based on yearly cycles. Further details on the model approach are provided in the electronic supplementary material (ESM).
Cascade of care for HCV infection. HCV hepatitis C virus, LTFU proportion lost to follow-up, PWID people who inject drugs, SVR proportion of patients who achieve sustained virological response, DCC decompensated cirrhosis, HCC hepatocellular carcinoma, LT liver transplant, PLT post-liver transplant, F0–F4 fibrosis stages 0–4
2.2 Population
The model population includes three key populations of interest for HCV, i.e. the general Spanish population, the migrant population, and people who inject drugs (PWID). Given the progress Spain has made recently in the treatment of HCV infection among incarcerated individuals and people living with human immunodeficiency virus (HIV), these populations were not considered explicitly [18].
The age distribution of the general and migrant population in Spain for 2015 (to coincide with the first availability of DAA treatment), was used to determine the model population flow and the net migration rates over time. Net migration by age and year were determined via yearly cohorts of 19-year-olds entering the model for years 2015–2019 [19]. For the purpose of the model, the 19-year-old cohort was considered to be reflective of the general population and no entry was made into the migrant population from the Spanish general population. Net migration rates were then determined for each year, by age distribution. Given the high prevalence of HCV among PWID, a rate of 0.42 per 1000 persons was applied to the overall population to include these individuals [20].
2.3 Clinical Inputs
2.3.1 Hepatitis C Virus (HCV) Prevalence
As HCV infection prevalence rates were not publicly available for the year 2015, the prevalence of active HCV infection in the general Spanish population from 2017 to 2018, based on a national prevalence study, was used [21]. A prevalence ratio was then applied to determine the prevalence in 2015 using a regional Spanish study that collected prevalence data in both 2017 and 2015 (calibrated ratio of 1.943) [9]. To determine prevalence among migrants, as the national prevalence study underreports this, an odds ratio (OR) representing the increased prevalence among the migrant population was applied (OR 2.8) [22]. Finally, the prevalence of HCV infection among Spanish PWID was calculated as the prevalence of HCV infection in PWIDs (58.5%) multiplied by the prevalence of PWID multiplied by the overall population of 16- to 64-year-olds [20, 23].
2.3.2 Disease Awareness and Progression
Awareness of infection impacted the rate of treatment, as aware individuals are more likely to be referred to treatment. The key awareness parameters among the three populations (general, migrant, PWID) is summarized in Table 1. The proportion of individuals referred to treatment was dependent on population; however, the proportion not referred was a single calibrated value for all populations (see the ESM for details on model calibration).
The proportion of individuals occupying each liver fibrosis stage for those who were aware of infection was taken from recent updates to the Spanish National HCV Plan documents (Table 1) [11]. As unaware individuals were assumed to be more likely to have mild disease, a ratio of 1.16 was applied to the individuals with fibrosis stage F0 or F1 in the aware population based on a recent Spanish study of HCV patients [24]. Unaware individuals with fibrosis stage F2–F4 were then re-weighted as per the aware population.
Patients who are chronically infected as well as those who are cured progress through liver disease at different rates. Therefore, the model used two different sets of transition probabilities for chronically infected patients and cured patients. The detailed transition probability matrix is presented in the ESM.
2.3.3 Diagnosis and Treatment
The model considered two sets of diagnosis rates. For patients with an initial HCV diagnosis, the model considered a primary diagnosis rate. For individuals who were modeled as being aware of infection but lost to follow-up, the probability of transitioning to treatment was based on a secondary diagnosis rate, i.e., the rate at which those who may have had a prior diagnosis of HCV infection in their lifetime were identified and then re-integrated into the health system to proceed with treatment.
The diagnosis rate of HCV infection in the general population was 0.037 and was determined via calibration (see the ESM for details). As primary diagnosis rates were not available for migrants in Spain, the diagnosis risk ratio for the migrant population versus those born in Spain was taken from a source on secondary diagnoses [9]. Using the risk ratio of 0.7 applied to the rate of the general population resulted in a rate for migrants of 0.026. A diagnosis rate of 0.089 for PWID was sourced from published literature, by taking the overall percentage of patients screened in the previous year, multiplied by the percentage of HCV RNA-positive patients who had an RNA test [25]. The secondary diagnosis rate in the model for the general population was 0.317. Using the same ratio of migrant to general population as above resulted in a calculated secondary diagnosis rate of 0.222. Due to a lack of data on secondary diagnosis rates in PWIDs, the same 0.089 primary rate sourced from the literature was also applied as the secondary rate [25].
Screening frequency was set in the model for each 10-year age bracket between the ages 20 and 79 years. In the status quo, no individual under 40 years of age was screened, regardless of population. In the increased testing scenario, the general population and migrants over the age of 40 years are set to be screened every 10 years; for PWID, everyone over the age of 20 years is screened once a year. In these populations, no screening was assumed to occur under the ages of 40 and 20, respectively. It was assumed in the base case that migrants are not screened on arrival, and the same screening frequencies were applied to this population as the general population. The resulting model calculations therefore take into account the age of patients in any given year and apply the appropriate screening costs based on the chosen time horizon. Alternative screening frequencies were tested through scenario analyses, including one where migrants are screened on arrival. It was assumed that once patients were diagnosed, there would be no differences in the proportion of patients treated between the scenarios.
In order to reflect current clinical practice in Spain, patients in the model do not immediately receive treatment when diagnosed, but have a delayed treatment start. As the mean number of days between diagnosis and treatment initiation was not available for each year of the model, multiple sources were used. The delay between diagnosis and treatment initiation in 2015 was taken from the time point at which 50,000 patients were treated, which was 570 days, as published by Spanish national statistics [11]. For the year 2017, a published source found the delay had decreased to 63 days [26]. For the year 2016, the average of these two treatment initiation delays was taken (316 days).
Treatment efficacy was dependent on disease stage and is summarized in Table 1. It was assumed that treatment regimens would be administered for 12 weeks [27].
2.3.4 Mortality
Both all-cause, age-specific mortality rates and excess mortality due to CHC were considered in the model (Table 1). General population mortality rates were taken from the National Statistics Institute of Spain; it was assumed that migrants had similar mortality rates. For PWID, a publication reporting cause-specific mortality among PWID was used, with a standardized mortality ratio of 16.37 applied to the general population rate [28, 29].
2.4 Cost Inputs
Costs are reported in 2021 Euros (€) and were inflated, where appropriate, using the Consumer Price Index of the National Statistics Institute of Spain [30]. Resource use in the model is driven by health state occupancy; the proportion of patients in each health state at any given time determines the resource use that is accrued by patients. Associated costs for these were sourced from the literature.
2.4.1 Health State Costs
Costs for the health states included in the model were taken from data from the published literature. As not all costs were reported with the same granularity used in our model, an average cost for stages F2 and F3, as reported in the literature, was used for F2, and the cost of F4 reported in the literature was used for both F3 and F4 stages, to reflect the appropriate disease severity; the approach was based on the recommendations of clinical experts. Health state costs and corresponding data sources are summarized in Table 1.
2.4.2 Treatment and Diagnosis Costs
The mean cost of DAA treatment was calculated as an average of two published studies, weighted by patient size, and was calculated to be €17,307, based on the list price of treatment [31, 32]. The cost of diagnosis in the model was based on the cost per patient screened and reflects the cost of antibody and ribonucleic acid (RNA) determination (€43) [33].
2.4.3 Extrahepatic Manifestation Costs
Adverse events outside the liver due to liver disease (in excess of the general population) may occur with CHC. In our analysis, these adverse events were not assumed to impact treatment efficacy or patients’ quality of life but resulted in additional costs for disease management. The mean cost of treating extrahepatic manifestations for the chronically infected and cured was determined by multiplying the extrahepatic manifestation costs (Table 1) by the excess proportion of extrahepatic manifestations to determine an annual cost.
2.4.4 Indirect Costs
Indirect costs via lost productivity were included in the model for those chronically infected (Table 1). These costs were based on estimates of the annual cost of lost productivity for those with CHC, alongside a matched uninfected control cohort [34].
2.5 Health State Utility Inputs
Utility values were incorporated into the model based on health state, and stratified by chronically infected and cured for fibrosis stage (Table 1). No adjustment was made for age.
2.6 Primary Analysis and Outcomes
The primary analysis aimed to quantify the economic benefits of increased HCV testing and treatment scenario compared with the current status quo in Spain. Primary outcomes of the model included total costs, total quality-adjusted life-years (QALYs), total life-years (LYs), the incremental cost per QALY gained, and incremental cost per LY gained.
2.7 Sensitivity and Scenario Analyses
Both one-way deterministic and probabilistic sensitivity analyses were conducted. In the one-way sensitivity analysis, most included parameters varied by 25%. In selected cases where inputs were deemed to be associated with lower levels of uncertainty, but variance was unreported in the source, a 5% variance was applied. More detail on the list of inputs and their variance is presented in Table 1. In the probabilistic analysis, 1000 iterations were run. In the absence of an officially accepted willingness-to-pay (WTP) threshold in Spain, two WTPs were explored (€25,000 and €30,000) [35].
Multiple scenario analyses were undertaken to test the robustness of the model. These included extending the model time horizon by 10 years to 2040, increasing the screening for the general population and migrants (while PWID remained the same), screening migrants for active HCV infection upon arrival into Spain, exploring the impact of decreasing DAA treatment costs by 10% every model year, exploring a decrease in the migration rate, and allowing patients to transition directly from F3 to advanced liver disease (decompensated cirrhosis/hepatocellular carcinoma).
3 Results
3.1 Base-Case Results
Under an increased testing and treatment scenario, the prevalence of active HCV infection decreased compared with the status quo. Projected prevalence with increased testing and treatment decreased to 0.13% in the year 2030. from 0.19% in 2021, while projected prevalence under the status quo was 0.16% in 2030. This reduction in prevalence translates to approximately 14,000 less infected individuals. Estimated active HCV incidence declined from 3.9 per 100,000 to 1.6 per 100,000 by 2030 under the increased testing and treatment scenario; a slower decline was projected with the status quo, with an estimated incidence of 2.4 per 100,000 by the year 2030. Cumulative HCV incidence and HCV-related mortality were both lower under the increased testing and treatment scenario (Table 2).
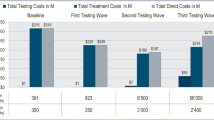
Total direct costs were €3.98 billionFootnote 1 and €3.23 billion for the increased testing and treatment scenario and the status quo, respectively. The largest contributors to direct costs were testing (€1.08 billion/€480 million), treatment (€780 million/€560 million) and management of extrahepatic manifestations (€1.17 billion/€1.19 billion for the increased scenario and the status quo, respectively). The higher direct costs were partially offset by lower indirect costs; total indirect costs were €214 million and €239 million for the increased scenario and the status quo, respectively. A breakdown of all costs is presented in Table 2.
The increased testing and treatment resulted in higher QALYs, resulting in an incremental cost-effectiveness ratio (ICER) of €13,291 per QALY. Net monetary benefit of the increased testing and treatment scenario was estimated to be €638 million and €911 million, at WTPs of €25,000 and €30,000 per QALY, respectively, compared with the status quo (Table 2).
3.2 Sensitivity Analysis
The deterministic sensitivity analysis found that the main drivers of the cost-effectiveness results were the cost of diagnosis, the proportion of HCV-infected people unaware of their infection in the general population, and the prevalence ratio of HCV between 2015 and 2017 (Fig. 3). The probabilistic sensitivity analysis showed that the increased testing and treatment scenario always resulted in positive incremental QALYs and positive incremental costs for the increased testing and treatment scenario compared with the status quo (Fig. 4). The ICER was below the €25,000 per QALY threshold in 96.60% of iterations and below the €30,000 threshold in 99.40% of iterations.
3.3 Scenario Analyses Results
The results of scenario analyses are presented in Table 3. When only direct costs were considered, the ICER increased to €13,761. Increasing testing time intervals to every 2 and 5 years resulted in ICERs of €29,217 and €18,761, respectively. When migrants in the model were to be screened at arrival in Spain, the ICER decreased to €7918.
4 Discussion
HCV infection is a global public health problem. This analysis found that scaling-up testing and treatment versus the status quo in Spain would result in approximately 13,000 fewer cases of HCV infection over the modeled time horizon, with an incremental cost per QALY of €13,291 and €13,761 per QALY from a societal and payer perspective, respectively. These results fall below the commonly accepted WTP of €25,000 in Spain. Furthermore, the net benefit of the scaled-up testing and treatment approach increased at higher WTPs. The model was most sensitive to the HCV prevalence ratio in the years 2015–2017 (used in calibration), the proportion of PWID, and age-specific prevalence inputs. These findings support the cost effectiveness of the strategy to scale-up testing and treatment for HCV in Spain.
These results are broadly in line with a previous economic simulation model that found increased testing and treatment compared with the status quo in a Spanish prison population was cost effective, resulting in an ICER of €9600 per QALY [33]. That analysis similarly adopted a 2015 baseline year to estimate the future HCV population, and had comparable model states (including an identical CHC disease state structure). However, it only considered a Spanish prison population, which our analysis did not include, somewhat limiting comparisons between studies. More recently, another study conducted in Spain compared three screening strategies with no screening among the general population and PWID [36]. The three screening strategies were (1) serological detection of antibodies against HCV; (2) dried blood spot test to detect antibodies against HCV; and (3) detection of RNA from HCV. The analysis found that all three strategies were cost effective, with ICERs of €13,633, €12,015 and €12,328/QALY, respectively. The authors concluded that the implementation of screening programs for HCV should not be limited by the financial resources needed, given their cost effectiveness.
As declared by the WHO and as reflected in the Spanish national HCV program, coordinated strategies are needed to achieve HCV elimination by 2030, even as new cases decline. There remains a large proportion of people who remain undetected by the Spanish health system, and thus are not seeking treatment [37]. This delay in seeking treatment is due to the time between infection and the presence of symptoms; these individuals may only enter the health system when symptoms or more serious complications arise [37].
This model has limitations. While HCV infection has been common in men who have sex with men, people living with HIV, and those in prison, these groups were not explicitly considered in the model. However, clinical opinion indicated that these populations are not drivers of HCV prevalence in Spain. The cost of scaling-up testing and treatment paradigms was not considered in the model beyond the cost of the test itself, which may have resulted in an underestimation of overall costs. However, by using list prices and not accounting for any price erosion due to generic treatments that are expected to enter the market over the modeled time horizon, the analysis likely overestimated drug costs. Furthermore, while lost productivity costs were included, these indirect cost data were limited in their availability and therefore might not be fully representative of a Spanish population. The relatively short time horizon utilized may be considered a limitation. However, given that the appearance of liver-related morbidity appears 10–20 years after diagnosis or infection, these results may be considered conservative. Furthermore, a scenario analysis exploring an increased time horizon resulted in a lower cost per QALY than the base case.
Additionally, the model did not account for age-dependent risk stratification, thus younger individuals who start in the model with very low HCV infection prevalence have their risk of HCV infection prevalence increase over time, which might not be observed in reality. However, as this parameter affects both the increased testing and treatment and status quo scenario, it was not considered to have a large impact on results. Finally, this model did not consider the effects of the ongoing COVID-19 pandemic on HCV testing and management, even though early data suggest elimination efforts have been negatively impacted [38,39,40]. Additional data are needed to robustly assess how the pandemic has impacted testing and management of HCV patients, along with elimination efforts, both globally and in Spain.
5 Conclusions
This analysis demonstrated that scaling-up testing and treatment with DAAs may be a cost-effective strategy to reduce the incidence and prevalence of CHC in Spain. Our findings provide further evidence that increasing access to testing and treatment may contribute to the global and Spanish public health goal of eliminating HCV and its associated liver-related morbidity and mortality.
Notes
Refers to 1,000 million throughout.
References
Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5(9):558–67.
World Health Organization. Key facts: hepatitis C 2020. https://www.who.int/news-room/fact-sheets/detail/hepatitis-c.
World Health Organization. Article: WHO updates guidance on hepatitis C infection 2022. https://www.who.int/news/item/24-06-2022-WHO-publishes-updated-guidance-on-hepatitis-C-infection.
World Health Organization. Hepatitis C in the WHO European Region—Fact Sheet 2021. Geneva: World Health Organization; 2021.
Estirado Gomez A, Justo Gil S, Limia A, Avellon A, Arce Arnaez A, Gonzalez-Rubio R, et al. Prevalence and undiagnosed fraction of hepatitis C infection in 2018 in Spain: results from a national population-based survey. Eur J Public Health. 2021;31(6):1117–22.
Lazarus JV, Bromberg DJ, Del Amo J, Norgaard O, Garcia-Samaniego J, Casellas A, et al. Hepatitis C prevalence among the migrant population in Spain: a systematic review and meta-analysis. Enferm Infecc Microbiol Clin (Engl Ed). 2019;37(4):222–30.
Rouger M. Hepatitis C management in Spain. 2015. healthcare-in-europecom.
Ramos H, Linares P, Badia E, Martin I, Gómez J, Almohalla C, et al. Re. Interferon-free treatments in patients with hepatitis C genotype 1–4 infections in a real world setting. World J Gastrointest Pharmacol Ther. 2017;8(2).
Juanbeltz R, Pérez-García A, Aguinaga A, Martínez-Baz I, Casado I, Burgui C, et al. Progress in the elimination of hepatitis C virus infection: a population-based cohort study in Spain. PLoS ONE. 2018;13(12): e0208554.
World Health Organization. WHO Hepatitis C Strategy. 2016. https://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/.
Spanish Ministry of Health SPaE. Plan Estratégico para el Abordaje de la Hepatitis C en el Sistema Nacional de Salud (PEAHC). 2020. https://www.mscbs.gob.es/ciudadanos/enfLesiones/enfTransmisibles/hepatitisC/PlanEstrategicoHEPATITISC/docs/Plan_Estrategico_Abordaje_Hepatitis_C_(PEAHC).pdf.
Corma-Gomez A, Pineda JA. Hepatitis C virus infection in Spain: challenges in the track to elimination. Enferm Infecc Microbiol Clin (Engl Ed). 2019;37(4):219–21.
Politi J, Guerras JM, Donat M, Belza MJ, Ronda E, Barrio G, et al. Favorable impact in hepatitis C-related mortality following free access to direct-acting antivirals in Spain. Hepatology. 2022;75(5):1247–56.
Stepanova M, Younossi ZM. Economic burden of hepatitis C infection. Clin Liver Dis. 2017;21(3):579–94.
Brennan A, Chick SE, Davies R. A taxonomy of model structures for economic evaluation of health technologies. Health Econ. 2006;15(12):1295–310.
Nelson RE, Deka R, Khader K, Stevens VW, Schweizer ML, Rubin MA. Dynamic transmission models for economic analysis applied to health care-associated infections: a review of the literature. Am J Infect Control. 2017;45(12):1382–7.
Lopez-Bastida J, Oliva J, Antonanzas F, Garcia-Altes A, Gisbert R, Mar J, et al. Spanish recommendations on economic evaluation of health technologies. Eur J Health Econ. 2010;11(5):513–20.
Marta Torrens. HCV elimination in Spain: overcoming challenges in high-risk populations. 2019. https://www.clinicaloptions.com/hepatitis/programs/hcv-treatment-as-prevention/clinicalthought/ct3/page-1.
Instituto Nacional de Estadítica. Población residente por fecha, sexo, grupo de edad y nacionalidad. 2002. https://ine.es/jaxiT3/Tabla.htm?t=9689.
European Monitoring Centre for Drugs and Drug Addiction. Statistical Bulleting 2020—search by country. 2020. https://www.emcdda.europa.eu/data/stats2020/search-by-country.
Ministerio de Sanidad Consumo y Bienestar Social. Grupo de trabajo del estudio de prevalencia de la infección por hepatitis C en población general en España; 2017–2018. Ministerio de Sanidad Consumo y Bienestar Social; 2019.
Crespo J, Cuadrado A, Perelló C, Cabezas J, Llerena S, Llorca J, et al. Epidemiology of hepatitis C virus infection in a country with universal access to direct-acting antiviral agents: data for designing a cost-effective elimination policy in Spain. J Viral Hepatitis. 2020;27(4):360–70.
Folch C, Casabona J, Espelt A, Majó X, Meroño M, Gonzalez V, et al. High prevalence and incidence of HIV and HCV among new injecting drug users with a large proportion of migrants—is prevention failing? Substance Use Misuse. 2016;51(2).
Morales-Arraez D, Nieto Bujalance Y, Diaz-Flores F, de Vera A, Jiménez A, Garcia Dopico JA, et al. Risk of liver fibrosis progression in patients with suboptimal diagnosis of hepatitis C virus infection. Eur J Gastroenterol Hepatol. 2020;32(4):528–34.
Nic An Riogh E, Swan D, McCombe G, O'Connor E, Avramovic G, Macías J, et al. Integrating hepatitis C care for at-risk groups (HepLink): baseline data from a multicentre feasibility study in primary and community care. J Antimicrob Chemother. 2019;74(Suppl 5):v31–v8.
Casas MP, García F, Freyre-Carrillo C, Montiel N, de la Iglesia A, Viciana I, et al. Towards the elimination of hepatitis C: implementation of reflex testing in Andalusia. Rev Esp Enferm Dig. 2020;112(7):515–9.
Emmanuel B, Wilson EM, O’Brien TR, Kottilil S, Lau G. Shortening the duration of therapy for chronic hepatitis C infection. Lancet Gastroenterol Hepatol. 2017;2(11):832–6.
Instituto Nacional de Estadítica. Resultados nacionales, por comunidades autónomas yy provincias. 2019.
Larney S, Tran LT, Leung J, Santo T Jr, Santomauro D, Hickman M, et al. All-cause and cause-specific mortality among people using extramedical opioids: a systematic review and meta-analysis. JAMA Psychiat. 2020;77(5):493–502.
Instituto Nacional de Estadística. Cálculo de variaciones del Indice de Precios de Consumo (sistema IPC base 2016). https://www.ine.es/varipc/.
Turnes J, García F, Diago M, Andrade RJ, Díaz P, Rubio-Rodríguez D, et al. Economic impact of health resource optimisation in the approach to patients with hepatitis C. Gastroenterol Hepatol. 2019;42(Suppl 1):26–33.
Garcia Herola A. Economic evaluation of an electronic health record alert by age in primary care for the detection of patients with chronic hepatitis C. ISPOR Europe 2019; 2–6 November 2019, Copenhagen.
Dalgic OO, Samur S, Spaulding AC, Llerena S, Cobo C, Ayer T, et al. Improved health outcomes from hepatitis C treatment scale-up in Spain’s prisons: a cost-effectiveness study. Sci Rep. 2019;9(1).
Younossi Z, Brown A, Buti M, Fagiuoli S, Mauss S, Rosenberg W, et al. Impact of eradicating hepatitis C virus on the work productivity of chronic hepatitis C (CH-C) patients: an economic model from five European countries. J Viral Hepatitis. 2016;23(3):217–26.
Vallejo-Torres L, García-Lorenzo B, Serrano-Aguilar P. Estimating a cost-effectiveness threshold for the Spanish NHS. Health Econ. 2018;27(4):746–61.
Nicolas Perez D, Morales Arraez DE, Castilla Rodriguez I, Gutierrez Nicolas F, Diaz-Flores Estevez F, de Vera GA, et al. Hepatitis C virus infection screening reduces mortality and is cost-effective independently of the intervention test. Rev Esp Enferm Dig. 2022;114(12):731–7.
Morales-Arraez D, Alonso-Larruga A, Diaz-Flores F, Garcia Dopico JA, de Vera A, Quintero E, et al. Predictive factors for not undergoing RNA testing in patients found to have hepatitis C serology and impact of an automatic alert. J Viral Hepat. 2019;26(9):1117–23.
Kaufman HW, Bull-Otterson L, Meyer WA 3rd, Huang X, Doshani M, Thompson WW, et al. Decreases in hepatitis C testing and treatment during the COVID-19 pandemic. Am J Prev Med. 2021;61(3):369–76.
Morales-Arraez D, Benitez-Zafra F, Diaz-Flores F, Medina-Alonso MJ, Santiago LG, Perez-Perez V, et al. Hepatitis C diagnosis slowdown in high-prevalence groups and using decentralised diagnostic strategies during the COVID-19 pandemic. Rev Esp Enferm Dig. 2023;115(4):175–80.
Sperring H, Ruiz-Mercado G, Schechter-Perkins EM. Impact of the 2020 COVID-19 pandemic on ambulatory hepatitis C testing. J Prim Care Community Health. 2020;11:2150132720969554.
Folch C, Saludes V, Reyes-Ureña J, Antuori A, Ibáñez N, Majó X, et al. The hepatitis C care cascade among people who inject drugs accessing harm reduction services in Catalonia: major gaps for migrants. Int J Drug Policy. 2021;90: 103057.
Barror S, Avramovic G, Oprea C, Surey J, Story A, Macías J, et al. HepCare Europe: a service innovation project. HepCheck: enhancing HCV identification and linkage to care for vulnerable populations through intensified outreach screening. A prospective multisite feasibility study. J Antimicrob Chemother. 2019;74(Suppl 5):v39–46.
EPCLUSA: summary of product characteristics.
Kamp WM, Sellers CM, Stein S, Lim JK, Kim HS. Impact of direct acting antivirals on survival in patients with chronic hepatitis C and hepatocellular carcinoma. Sci Rep. 2019;9(1):17081.
Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic manifestations of hepatitis C: a meta-analysis of prevalence, quality of life, and economic burden. Gastroenterology. 2016;150(7):1599–608.
Cacoub P, Buggisch P, Carrión JA, Cooke GS, Zignego AL, Beckerman R, et al. Direct medical costs associated with the extrahepatic manifestations of hepatitis C infection in Europe. J Viral Hepatitis. 2018;25(7):811–7.
Ruggeri M, Romano F, Basile M, Coretti S, Rolli FR, Drago C, et al. Cost-effectiveness analysis of early treatment of chronic HCV with sofosbuvir/velpatasvir in Italy. Appl Health Econ Health Policy. 2018;16(5):711–22.
Buti M, San Miguel R, Brosa M, Cabasés JM, Medina M, Casado MA, et al. Estimating the impact of hepatitis C virus therapy on future liver-related morbidity, mortality and costs related to chronic hepatitis C. J Hepatol. 2005;42(5):639–45.
Tinoco-González J, Suárez-Artacho G, Bernal-Bellido C, Cepeda-Franco C, Ramallo-Solis I, Marín-Gómez L, et al. Analysis of the first 1000 liver transplants in Virgen del Rocío Hospital. Transpl Proc. 2016;48(9):2973–6.
Buti M, Gros B, Oyagüez I, Andrade RJ, Serra MA, Turnes J, et al. Cost-utility analysis of triple therapy with telaprevir in treatment-naïve hepatitis C patients. Farmacia Hospitalaria : Organo Oficial de Expresion Cientifica de la Sociedad Espanola de Farmacia Hospitalaria. 2014;38(5):418–29.
San Miguel R, Gimeno-Ballester V, Blázquez A, Mar J. Cost-effectiveness analysis of sofosbuvir-based regimens for chronic hepatitis C. Gut. 2015;64(8):1277–88.
Mar J, Ibarrondo O, Martínez-Baz I, Juanbeltz R, San Miguel RIC, et al. Economic evaluation of a population strategy for the treatment of chronic hepatitis C with direct-acting antivirals. Revista española de enfermedades digestivas: organo oficial de la Sociedad Española de Patología Digestiva. 2018;110(10):621–8.
Juanbeltz R, Castilla J, Martínez-Baz I, O’Leary A, Sarobe M, San MR. Health-related quality of life in hepatitis C patients who achieve sustained virological response to direct-acting antivirals: a comparison with the general population. Qual Life Res. 2019;28(6):1477–84.
Buchanan-Hughes AM, Buti M, Hanman K, Langford B, Wright M, Eddowes LA. Health state utility values measured using the EuroQol 5-dimensions questionnaire in adults with chronic hepatitis C: a systematic literature review and meta-analysis. Qual Life Res. 2019;28(2):297–319.
Pol S, Chevalier J, Brancoux S, Perry R, Milligan G, Gaudian A. Health-related quality of life and utility values in chronic hepatitis C patients: a cross-sectional study in France, the UK and Germany. J Hepatol. 2015;62.
Acknowledgements
Medical writing and editorial assistance were provided by Lianne Barnieh of Maple Health Group.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Jose Luis Calleja has received consultant and lecturer honoraria from Gilead Sciences, AbbVie, Roche, MSD and Intercept Pharmaceuticals. Ankita Kaushik is an employee and stockholder of Gilead Sciences. Alon Yehoshua is a former employee of Gilead Sciences. Manuel Hernandez-Guerra has received research grants from AbbVie and Gilead Sciences, and has participated in consultant advisories for Bayer, Orphalan and Intercept Pharmaceuticals. Rob Blissett and Adam Igloi-Nagy are paid employees of Maple Health Group, which provides consulting services to Gilead Sciences. Jaime Espin has no competing interests to declare that are relevant to the contents of this article.
Funding
This study was funded by Gilead Sciences.
Availability of data and material
All secondary data generated or analyzed during this study are included in the published article or its supplementary materials.
Author contributions
RB and AIN conceptualized and built the model. JLC, JE, AK, MHG, and AY reviewed and critiqued the model and its results. AIN and RB wrote the manuscript, with review and input from JLC, JE, AK, MHG, and AY. All authors read and approved the final manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Model availability
Model inputs were sourced from data in the public domain. The economic model may be requested from the authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Calleja, J.L., Espin, J., Kaushik, A. et al. The Efficiency of Increased HCV Testing and Treatment Strategies in Spain to Achieve Elimination Goals. PharmacoEconomics Open 8, 221–233 (2024). https://doi.org/10.1007/s41669-023-00458-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-023-00458-3