Abstract
Background
Nonalcoholic steatohepatitis (NASH) is characterized by inflammation and hepatocellular damage caused by accumulation of fat in the liver. Resmetirom (MGL-3196) is an orally administered, small-molecule, liver-targeted, selective thyroid hormone receptor-β agonist. This early analysis explored the potential cost effectiveness of resmetirom for the treatment of NASH from a US commercial payer perspective.
Methods
An early economic model was developed to reflect the clinical pathways typically followed by patients with NASH and liver fibrosis. Use of resmetirom, compared with placebo, was assessed. The Markov model structure was informed by a previous modeling study and a randomized, double-blind, placebo-controlled, phase II trial of resmetirom. Costs and outcomes were assessed over a lifetime time horizon with results presented in terms of cost per quality-adjusted life-year (QALY) gained.
Results
Resmetirom treatment resulted in increased costs of US$66,764 per patient, while increasing QALYs by 1.24. The incremental cost-effectiveness ratio was US$53,929 per QALY gained, indicating resmetirom treatment would potentially be cost effective at a willingness-to-pay (WTP) threshold of US$100,000. Results indicated that resmetirom would reduce the lifetime number of cases of decompensated cirrhosis (− 87), hepatocellular carcinoma (− 59), and liver transplants (− 30) per 1,000 patients compared with placebo. Resmetirom treatment remained cost effective at a US$100,000 WTP threshold up to a daily price point of US$72.00.
Conclusion
Resmetirom is a potentially cost-effective treatment option for patients with NASH and liver fibrosis based on an analysis performed from a US commercial payer perspective. Future economic analyses of the technology should, however, focus on overcoming the limitations of existing modeling methodology.
Similar content being viewed by others
Economic modeling using phase II data indicates that resmetirom treatment has an 86.20% probability of being cost effective at a willingness-to-pay threshold of US$100,000 per quality-adjusted life-year from a US commercial payer perspective. |
Resmetirom is likely to be cost-incurring to treat nonalcoholic steatohepatitis (NASH) (+US$66,764 per patient), given the lack of available therapies, but is projected to be clinically beneficial and overall cost effective. |
Future economic analyses of resmetirom treatment should focus on overcoming limitations associated with current NASH modeling, including early disease activity or NASH resolution, and heterogeneity of populations in terms of comorbidities and disease severity. |
1 Introduction
Nonalcoholic steatohepatitis (NASH) is characterized by inflammation and hepatocellular damage caused by accumulation of lipotoxic fat in the liver [1]. NASH is the progressive form of nonalcoholic fatty liver disease (NAFLD) and is more prevalent among individuals who are overweight or obese [2]. NAFLD develops in four main stages which increase with severity. The first stage is nonalcoholic fatty liver (NAFL), or isolated hepatic steatosis, in which a build-up of fat in the liver cells occurs. The second stage is NASH with no, or early, fibrosis, which is a more serious form of NAFLD, where lipotoxic species cause inflammation and hepatocellular damage. The third stage is NASH with significant fibrosis, which is characterized by accumulating scar tissue in the liver due to persistent inflammation (although liver functionality remains normal). Finally, the progression of NASH with fibrosis may lead to cirrhosis, which in turn may lead to decompensated cirrhosis (DCC), liver failure, and hepatocellular carcinoma (HCC) [2]. Progression of disease has been shown to be more rapid amongst patients with NASH, and amongst those with comorbidities such as type 2 diabetes mellitus (T2D), compared with those with a less progressive form of NAFLD (i.e., NAFL) [3].
NAFLD is one of the most significant liver diseases worldwide affecting both adults and children [4]. Its prevalence is correlated with increasing age, with rates highest amongst males aged 40–65 years. The global prevalence of NAFLD is estimated to be 24%, with high rates reported in both the United States (US, 24%) and in Europe (23%) [4]. Of the prevalent NAFLD cases in the US, approximately 20–30% of these (17–25 million Americans) are thought to have NASH [5]; in England, NASH is estimated to affect up to 5% of the general population [2]. With rising obesity rates, the global prevalence of NAFLD and NASH is expected to continue to increase. A 2018 US-based study by Estes et al. forecast that prevalent NAFLD cases will increase by 21% to 100.9 million between 2015 and 2030, while prevalent NASH cases will increase by 63% to 27 million cases over the same period [6].
As well as having a detrimental impact on patient quality of life, multiple studies have highlighted the significant healthcare costs and utilization of health services associated with NAFLD and NASH [7,8,9,10,11,12]. A 2020 US-based study by Younossi et al. described a model developed to estimate economic burden and clinical outcomes associated with the condition over a 20-year time horizon [7]. Their study indicated the total cost of NASH with T2D will be US$667.9 billion between 2017 and 2037, with US$504.2 billion (75.5%) related to T2D management and US$163.7 billion (24.5%) related to NASH care. Additionally, NASH, in combination with T2D, will potentially be responsible for 64,900 liver transplants (LTs), 812,000 liver-related deaths, 1.37 million cardiovascular (CV) deaths, 1.27 million DCC person-years, and 479,000 HCC person-years over the next 20 years in the US [7]. Further global studies have demonstrated the significant costs associated with the condition, particularly in the latter stages of disease [8,9,10,11,12].
Magnetic resonance imaging-derived proton density fat fraction (MRI-PDFF) is a quantitative imaging biomarker that allows for accurate assessment of fat in the entire liver and reflects the concentration of mobile triglycerides within the tissue [13]. Previous studies have demonstrated that a ≥ 30% relative reduction in MRI-PDFF is associated with higher odds of histologic response and NASH resolution [14], and MRI-PDFF serves as a surrogate to liver biopsy for assessing treatment response in NASH phase II trials [13]. The US Food and Drug Administration (FDA) recommends resolution of steatohepatitis and no worsening of fibrosis, and fibrosis improvement without worsening of NASH, as endpoints to support accelerated approval for NASH therapeutics, since it is likely to predict clinical benefit [15]. For economic modeling purposes, the FDA-recommended endpoint could be utilized as a composite endpoint as the independent association of NASH disease activity and improvement of liver or CV outcomes is not currently captured in economic models.
Due to the significant costs and association with poor clinical outcomes, appropriate treatment of NASH is essential to slow progression to more severe disease. Typically, treatment involves lifestyle adjustments aimed at weight loss such as exercising more regularly and improving diet, while a LT may be required in the event of severe cirrhosis [16]. Currently, there are no approved treatments for NASH.
Resmetirom (MGL-3196) is an orally administered, small-molecule, liver-targeted, selective thyroid hormone receptor (THR)-β agonist which has shown promising results in a randomized, double-blind, placebo-controlled, phase II trial in patients with NASH and fibrosis. Preclinical, toxicology, and phase I/II clinical data suggest resmetirom has an attractive, differentiated profile for the treatment of NASH [17, 18]. In a randomized, placebo-controlled, 36-week, phase II, serial liver biopsy trial in patients with NASH and fibrosis, resmetirom demonstrated statistically significant reductions compared with placebo in MRI-PDFF, liver enzymes, markers of inflammation, and fibrosis. Resmetirom-treated patients also had improvements in NASH on liver biopsy compared with placebo-treated patients, with a significantly greater proportion of patients achieving NASH resolution (27% vs 6%, respectively) [18]. Also, in a noninvasively identified NASH population, open-label treatment with resmetirom 100 mg daily resulted in a 53% reduction in MRI-PDFF, 23% reduction in low-density lipoprotein-cholesterol (LDL-C), 22% reduction in apolipoprotein B, 39% reduction in triglycerides, and 39% reduction in lipoprotein(a) after 52 weeks [19].
Following promising clinical results, this early analysis describes an economic model developed to assess the relative cost effectiveness of resmetirom (a potential new technology) compared with placebo from a US commercial payer perspective, alongside a pricing analysis. Limitations associated with existing NASH modeling methodology are also comprehensively discussed, in the context of future research on the economic viability of resmetirom.
2 Methods
An economic decision model was developed in Microsoft Excel to assess the relative cost effectiveness of resmetirom compared with placebo. A Markov model, with a 1-year cycle length, was selected to simulate both treatment strategies, including the clinical pathways of a patient with NASH with fibrosis as well as potential CV events (myocardial infarction [MI] and stroke); other hepatic complications including DCC, HCC, and LT; hospitalizations; and mortality. The model structure was informed by the NASH model previously developed by the Institute for Clinical and Economic Review (ICER), which focused on the use of obeticholic acid for the treatment of NASH with fibrosis [20]. This model, as well as other published models including Younossi et al. [7], primarily focused on fibrosis health states within their model structure, which is a commonly used approach in models of NASH. The appropriateness of the ICER NASH model for this analysis was confirmed by clinical expert input [20]. When necessary, experts were used to confirm the clinical accuracy and plausibility of the modeling assumptions and results shown in the model. However, a specific consensus solicitation approach was not applied during the input process, with views being consistent across clinical experts.
The model presented in this analysis is structurally and methodologically interchangeable with the ICER NASH model but contains resmetirom-specific analyses utilizing appropriate data.
The analysis was performed from a US commercial payer perspective over a lifetime time horizon to assess the long-term clinical and economic impact of resmetirom treatment. The modeling methods followed the Professional Society for Health Economics and Outcomes Research (ISPOR)’s ‘Principles of Good Practice for Decision Analytic Modeling in Health-Care Evaluation’ [21]. Costs and benefits were discounted at a rate of 3% as recommended by ICER in the US [22].
2.1 Model Overview
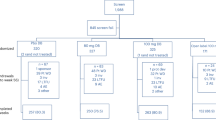
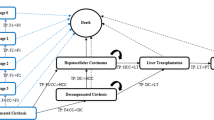
The model structure is presented in Fig. 1. A Markov model structure was used to allow for the transition of patients between relevant health states. The model was composed of two sub-models (‘no prior CV event’ and ‘prior CV event’) with equivalent liver disease-specific state transition probabilities to account for the occurrence of MI and stroke. Each sub-model allows for transitions among no fibrosis (F0) and fibrosis (F1–F3) stages, compensated cirrhosis (CC or F4), DCC, HCC, post-liver transplant (PLT), and death; the costs and health impacts of undergoing LT are assessed with the transition to PLT. Patients can transition from any of the alive health states to death from all causes including CC, DCC, HCC, LT, CV events, or background mortality.
The transition from the first sub-model (no prior CV event) to the second sub-model (prior CV event) is driven by the first occurrence of a CV event. The costs, quality of life, and survival associated with the first CV event is assessed with the transition. Patients with NASH who enter the prior CV event sub-model are at increased risk of subsequent CV events and mortality. The worsening or improvement in the patient’s condition informs their transition through the model. This is modeled according to the stage of fibrosis (NASH-F0, F1, F2, F3) progressing to CC, DCC, HCC, and the need to undergo LT. Simulated patients are subject to experiencing CV events and mortality at any stage of disease progression.
Patients in the model are assumed to be an average age of 50 years at model entry (based on information from the resmetirom phase II trial in patients with NASH [18]) and receive either resmetirom 100 mg or placebo daily. Although patients in the resmetirom phase II trial were on dosages ranging from 40 mg to 100 mg and reported efficacy of resmetirom based on these diverse doses [18], from a cost perspective and applying a conservative approach, the price of different doses was assumed to be the same as for 100 mg daily. This assumption was made as a preliminary cost analysis was only available for resmetirom 100 mg.
2.2 Model Inputs
All model inputs (clinical, utility, and cost parameters) are outlined in the following sections. Model input values, as well as assigned distributions and ranges of values, are presented in Table 1.
2.2.1 Clinical Effectiveness Parameters
Clinical data were primarily derived from three main sources and the baseline characteristics of the modeled population were obtained from the resmetirom phase II trial [18]. This was a 36-week, randomized, double-blind, placebo-controlled trial performed at 25 centers in the US, with a primary endpoint of 12-week relative change in MRI-PDFF, and secondary endpoints including relative hepatic fat reduction at 36 weeks and proportion of patients with a 2-point reduction in NAFLD activity score (NAS). The initial distribution of patients across fibrosis health states, and the clinical complication rate amongst patients on/off resmetirom, were informed by data from the resmetirom phase II trial [18]. Clinical complications modeled included the adverse occurrence of alanine aminotransferase (ALT), gastrointestinal (GI) disorders, and gamma-glutamyl transferase (GGT). Additional data related to change in LDL-C, high-density lipoprotein cholesterol (HDL-C), and triglycerides as well as reduction in systolic blood pressure (SBP) and absolute change in LDL-C at 36 weeks with/without resmetirom were also sourced from data from the resmetirom phase II trial [18].
Data on the effectiveness of resmetirom were sourced from the study by Loomba et al. [23], which discussed MRI-PDFF treatment response criteria in NASH based on a secondary analysis of the data from the resmetirom phase II trial [18], and which reported results of additional analyses performed. Their analysis reported the percentage of MRI-PDFF responders (≥ 30% reduction) and non-responders (< 30%) who would be fibrosis responders (i.e., regression in health state) as well as the proportion of patients who would progress (worsen) fibrosis health state and not change state. These data were used to estimate the average percentage of patients who would regress, progress, or not change on resmetirom, based on whether or not they achieved an MRI-PDFF response. Using the biopsy results from the phase II trial and the corresponding MRI-PDFF data, it was estimated that approximately 32% of patients in the resmetirom arm achieved regression in fibrosis health state at 36 weeks with an approximate 59% estimated to not change health state [23]. Therefore, approximately 9% of patients were estimated to progress (worsen) in fibrosis health state (i.e., the remaining percentage of patients who did not achieve regression or no change in health state). The relationship between MRI-PDFF and NAS has previously been highlighted in a study by Loomba [24], with information from Brunt et al. [25] on the link between NAS and fibrosis justifying the adopted approach to estimating the proportion of patients ‘improving’ and ‘worsening’ in each model cycle. Information on fibrosis improvement, or regression, in the placebo arm was derived from the resmetirom phase II trial [18], with data on the proportion of patients worsening or progressing in fibrosis health state on placebo sourced from the ICER NASH model based on the proportion in their control arm who worsened (21%) [20]. Uncertainty in these parameters was explored in sensitivity analysis, with wide variation in values applied. Further treatment effectiveness information including the average length of time over which patients would receive treatment (30 years) and the percentage of the population who would still be compliant on resmetirom on a yearly basis (95%, applied to the compliance percentage from the previous cycle) were based on assumptions informed by expert clinical input. The experts involved in providing input during the modeling process are outlined in the ‘Declarations’ section of the manuscript.
Finally, transition probabilities to determine the health states to which patients would move when improving or worsening (i.e., the transition matrix) were sourced from the ICER NASH model [20]. Their analysis used data from a previous systematic review and meta-analysis performed by Singh et al. to inform the stage-specific transitions when ‘improving’ or ‘worsening’ in the early stages of fibrosis [3]. The ICER NASH model also provided data on the annual transition probabilities in the latter stages of fibrosis as well as data on the annual probability of death based on information from a number of previous studies [26,27,28,29] (in addition to a study by Rustgi et al. [30], which provided data on the transition probabilities for patients moving from DCC and HCC to receive LT, and a study by Lidgren et al., which reported the probability of death associated with LT [31]). Conservatively, the model assumed that there was no possibility of transitioning from F4 to less severe fibrosis health states (i.e., F0–F3), which is clinically accepted [32, 33].
The 10-year CV risk was calculated in the model using the Framingham CV risk calculator, which uses data on patient’s sex, age, and clinical characteristics to calculate risk [34]. Baseline patient characteristics, primarily derived from the resmetirom phase II trial [18], on sex, age, total cholesterol, HDL-C, and T2D status were used as input values in the calculation and 10-year CV risk in each arm of the model was estimated. The only patient characteristics not reported in the resmetirom phase II trial required for the estimation of CV risk were the initial proportion of patients who smoke (which was informed by data from the Center for Disease Control and Prevention [35]), and baseline SBP (mmHg) (which was assumed based on expert clinical input). Data on the annual increase in CV risk used in the model was informed by expert clinical input. Additional data related to the occurrence of CV events were sourced from the ICER NASH model [20]. It included data on the proportion of CV events that are MI versus stroke and the proportion of fatal MIs and strokes (which were sourced from Virani et al. [36]) as well as the risk ratio of a CV event based on whether LDL-C changes over time [20], which was sourced from Silverman et al. [37] (data on LDL-C sourced from the resmetirom phase II trial [18]). Additionally, the ICER NASH model reported the relative risk of a recurrent CV event amongst patients who had experienced a prior event [20], which they sourced from Jernberg et al. [38]. The presented approach was followed in the base-case analysis, with alternative assumptions related to CV risk explored in scenario analyses (presented later). The base-case analysis also assumes that patients have had no prior CV history directly associated with NASH. All clinical input parameters can be seen in Table 1.
2.2.2 Utilities
Utility values for modeled fibrosis health states were informed by data from the Global Assessment of the Impact of Pain (GAIN) study [11], while utility values associated with CC, DCC, and HCC were sourced from a previous cost-effectiveness analysis [39]. The GAIN study was a prevalence-based burden of illness study across Europe (France, Germany, Italy, Spain, and the UK) and the USA conducted among patients found to have NASH on liver biopsy. Of the included patients, 767 (20%) provided information on indirect costs and quality of life estimated using the EQ-5D [11]. The previous cost-effectiveness analysis, performed by Younossi et al. [39], sourced utility values from prior studies by Dan and Younossi [40] and Hsu et al. [41]. The study by Hsu et al. looked at health utilities and psychometric quality of life in patients with early- and late-stage hepatitis C virus infection. The study was conducted at six tertiary care sites in the metropolitan area of Vancouver, British Columbia, Canada, with the SF-36 Health Survey Version 2 (SF36v2) used to estimate utility values [41]. Details of the method of estimating utility values are limited in the study by Dan and Younossi [40], although use of the associated values for CC and DCC utilized in the established economic model by Younossi et al. [39] indicate that they are reasonable to include in this analysis. The utility values for patients in the LT and PLT states were assumed to be the same as those of CC patients and of patients with stage 3 fibrosis, respectively, based on an assumption informed by evidence in the review article by Yang et al., which explored long-term quality of life among patients who had undergone primary LT [42]. Disutility values associated with MI, stroke, and prior CV events were sourced from a study exploring EQ-5D scores amongst patients with chronic conditions in the US [43]. All utility values are presented in Table 1.
2.2.3 Costs
As the unit cost for daily use of resmetirom is still under consideration and the analysis aimed to explore price thresholds, an arbitrary value per 100 mg was advised by Madrigal Pharmaceuticals, Inc., and was varied in sensitivity analysis [20]. As mentioned previously, patients in the resmetirom phase II trial [18] were on a range of dosages (40–100 mg), but this modeling study applies a 100-mg unit cost. Costs of modeled health states were obtained from the ICER NASH model [20], which had utilized state-specific costs from Younossi et al. [39], CV disease costs from a previous cost-effectiveness analysis [44], and CV death costs from an earlier costing study in the area of CV disease [45]. Costs included in the ICER NASH model [20] were inflated to a 2021 price year (US$) using the Campbell Collaboration tool for currency conversion and inflation (implied inflation factor = 1.02) [46]. Costs of ALT and GI disorders were sourced from Chirikov et al. [47] and were inflated with the same tool using an implied inflation factor of 1.11 [46]. Due to data limitations, it was assumed that the cost of adverse GGT was the same as ALT. All cost values are presented in Table 1.
2.3 Analysis
2.3.1 Base-Case Analysis
A cost-utility analysis was performed, with a focus on cost per quality-adjusted life-year (QALY) gained associated with introduction of resmetirom for the treatment of NASH and fibrosis. In addition, the model estimated the change in the number of patients experiencing CV events (MI and stroke) and hepatic complications following introduction of resmetirom treatment (based on an assumed 1000 patient population size).
Base-case results are presented probabilistically. The probabilistic analysis was performed as a Monte Carlo simulation, whereby an appropriate distribution was assigned to individual model parameters (allowing for a plausible value from that distribution to be selected), and 5000 model iterations were run. Probabilistic outputs were produced, including a cost-effectiveness plane (scatterplot) of results and a cost-effectiveness acceptability curve (CEAC) to show the likelihood of the intervention being cost effective across a range of willingness-to-pay (WTP) thresholds. Based on a US reference case, a WTP threshold of US$100,000/QALY was applied to assess cost effectiveness of resmetirom treatment in the base-case analysis [48].
Internal validation of the model results was performed by comparing results with those produced in the ICER NASH model [20] with external validation performed by comparing results with a comparable cost-effectiveness analysis in the area of NAFLD [30].
2.3.2 One-Way Sensitivity Analysis
To explore uncertainty in the model results, a deterministic sensitivity analysis (DSA) was performed. The DSA involved varying individual model parameters to explore their impact on the overall results. Results were estimated based on the 95% confidence interval of individual parameters or based on ±20% variations.
2.3.3 Threshold Analysis
Price threshold analyses were performed to determine the daily drug treatment cost at which resmetirom would still be considered cost effective at US$50,000, US$100,000, and US$150,000 WTP thresholds. This analysis was performed probabilistically.
2.3.4 Scenario Analyses
Two separate scenario analyses were performed to explore the impact of alternative approaches to estimating CV risk using patient-level data from the resmetirom phase II trial [18]. In the first scenario, a pooled CV risk for both arms (10-year risk of 8.86% for each) was used based on the assumption that NAFLD and NASH may be independent risk factors for CV disease even after controlling for the high rates of baseline risk using patient-level data from the resmetirom phase II trial [49]. In the second scenario, data on the average 10-year CV risk in patients older than 40 years of age in the resmetirom arm (9.01%) and placebo arm (8.47%) at baseline were used, based on patient-level data from the resmetirom phase II trial [49]. A third scenario analysis was performed to explore the impact of adjusting the distribution of patients across initial fibrosis health states. In this analysis, it was assumed that only the more severe fibrosis cases would be included, with an assumed distribution of 58% and 42% of patients across the F2 and F3 health states, respectively (distribution informed by the proportional distribution in the base-case analysis). Results of these analyses are presented in the following section.
3 Results
3.1 Base-Case Analysis
Results of the base-case probabilistic analysis are show in Table 2. Results indicate that costs per patient in the resmetirom arm are US$348,432 over a lifetime time horizon, which is US$66,764 more costly than placebo (US$281,668) on an individual patient basis. The results of the base-case analysis also show that resmetirom (12.75) leads to more QALYs than placebo (11.52) over a lifetime (incremental QALYs gained of 1.24). The incremental cost-effectiveness ratio is US$53,929 per QALY gained, which is below the US$100,000 per QALY threshold of cost effectiveness in the US.
The base-case analysis also examined the number of clinical events that may be avoided through use of resmetirom amongst this patient population (based on a hypothetical cohort of 1000 patients). Table 2 shows that the number of DCC events (− 87), HCC events (− 59), and LTs (− 30) would all be reduced over a lifetime through use of resmetirom treatment.
Further results of the base-case probabilistic analysis are shown in Fig. 2. The cost-effectiveness plane shows that the large majority of points are in the north-east quadrant of the plane, indicating that the intervention is likely to be costlier, but also more effective, than placebo. The CEAC shows that the probability of the intervention being cost effective increases as the WTP threshold increases, and that it has an 86.20% probability of being cost effective at a WTP threshold of US$100,000.
3.2 One-Way Sensitivity Analysis
One-way DSA was performed to explore the impact of individual model parameter variation on the overall results. Figure 3 is a tornado diagram showing the impact that each parameter variation (increase and decrease) has on the base-case net monetary benefit (NMB) (US$60,063) of resmetirom. NMB represents the value of the intervention in monetary terms, calculated as (incremental benefit × threshold) − incremental cost. Results indicate that the main drivers of the model results are the proportion of patients achieving improvement in fibrosis at 36 weeks with resmetirom and placebo. Additionally, the proportion of patients achieving no change in fibrosis at 36 weeks with resmetirom and placebo, and the utility values associated with the early stages of NASH, are key drivers of the model results.
3.3 Threshold Analysis
Threshold analyses at US$50,000, US$100,000, and US$150,000 WTP thresholds were performed probabilistically to determine the daily price at which resmetirom would still be considered cost effective. Results indicate that resmetirom would be cost effective at a daily price of US$50.35 (US$50,000 WTP threshold), US$72.00 (US$100,000 WTP threshold), and US$93.64 (US$150,000 WTP threshold), depending on the selected WTP threshold (Table 3).
3.4 Scenario Analyses
Three separate scenario analyses were performed to examine different assumptions, two of which were related to CV risk (see Sect. 2.3.4). In the base-case analysis, CV risk in each arm was based on risk adjustment using the Framingham tool. Alternative approaches were taken in scenario analysis 1 (assumed identical CV risk in each arm) and scenario analysis 2 (assumed differing baseline CV risk for each arm) (Table S1, Appendix 1 in the electronic supplementary material). The results are largely consistent with the base-case analysis, with incremental cost-effectiveness ratios of US$58,355 and US$59,716 per QALY gained for scenario analyses 1 and 2, respectively (base-case analysis = US$53,929 per QALY gained). In scenario analysis 3, where it was assumed that all patients in the model were initially distributed between the F2 and F3 health states only, the incremental cost-effectiveness ratio is US$51,862 per QALY gained, suggesting a marginal improvement in potential cost effectiveness compared with the base-case analysis.
4 Discussion
An economic model was developed to consider the costs and health outcomes associated with the introduction of resmetirom for the treatment of NASH and fibrosis. Base-case results indicate that resmetirom treatment is cost-incurring, but more effective, over a lifetime time horizon, with an incremental cost-effectiveness ratio of US$53,929 per QALY gained. Results also indicate that resmetirom would reduce the number of HCC, DCC, and LT events incurred, while probabilistic results indicate that the treatment would potentially have an 86.20% probability of being cost effective at a WTP threshold of US$100,000. Results of the one-way DSA show that those parameters which are most impactful on the model are the proportion of patients achieving improvement in fibrosis at 36 weeks as well the utility values associated with the early stages of NASH. A scenario analysis exploring an initial distribution of all modeled patients across F2 and F3 patients did not result in a major difference compared with the base-case results, although the incremental cost-effectiveness ratio was reduced to US$51,862 per QALY gained, indicating that targeting treatment to more severe cases has the potential to improve value for money. This result was largely consistent with the analysis performed by Rustgi et al. [30], which explored the cost effectiveness of a hypothetical treatment for NAFLD. Their scenario analyses also explored the impact of targeting treatment to specific stages, and found little change from the base-case results.
Probabilistic threshold analyses related to the price of resmetirom were also performed, which show that the maximum daily price at which resmetirom would remain cost effective ranges from US$50.35 (US$50,000 WTP threshold) to US$93.64 (US$150,000 WTP threshold). The economic model has been largely informed by data from the resmetirom phase II trial in patients with NASH and stage 1–3 fibrosis [18]; in particular, the baseline patient characteristics and the initial distribution of patients across fibrosis health states used in the model. Multiple studies have highlighted the global economic burden associated with NASH and NAFLD [7,8,9,10,11,12], and a clinically beneficial and potentially cost-effective treatment such as resmetirom may reduce this burden.
The economic model structure presented has been informed by the ICER NASH model, which looked at the lifetime cost effectiveness of obeticholic acid, compared with current standard care, for adults with NASH with fibrosis [20]. Their analysis used interim data from the REGENERATE trial-reported secondary outcomes for ‘improvement’ and ‘no change’ to inform the transition probabilities between modeled health states [50]. These data were used to inform the proportion of patients improving, worsening, and staying in the same health state, with data from the results of a meta-analysis of fibrosis progression in NAFLD versus NASH used to inform the early fibrosis stage transitions in the event that patients improved or worsened [3]. This same approach and model structure was followed in our analysis of resmetirom, with treatment-specific effectiveness data from Loomba et al. used to inform the percentage of patients improving and worsening in each cycle of the model [23], and the same data from Singh et al. used to model subsequent stage transitions [3], with additional sources used to inform progression in the more severe stages of fibrosis [26,27,28,29,30]. Given the importance of the ICER NASH model in informing our own model, its limitations should be highlighted [20]. These limitations, which can be applied to our own analysis as well as a large number of NASH models, are presented below.
Firstly, the model assumed that there is a linear disease progression associated with fibrosis rates. However, as seen in the study by Singh et al., the rate of fibrosis progression can vary between subgroups of patients with NAFLD, NAFL, and NASH [3]. Additionally, Singh et al. identified two subsets of patients with NAFLD in their analysis: rapid progressors (21%), who progressed from stage 0 fibrosis to stage 3 or 4 over a mean (± SD) follow-up period of 5.9 (± 3.7) years, and patients who showed slow progression in their fibrosis stage at the follow-up liver biopsy, usually only by one or two stages [3]. Therefore, to assume that all patients progress through the stages of fibrosis at the same rate, without accounting for variability in disease progression, appears to be an inherent flaw of this modeling approach and a limitation that should be addressed in future models of NASH. Data from Singh et al. [3] are commonly used in economic models of NASH to inform fibrosis stage transition probabilities [20, 39, 51]. However, the systematic review from Singh et al. only included literature up until 2013, and is therefore now in need of updating, while the meta-analysis performed included data from only 11 observational studies [3]. Limitations of the Singh et al. analysis in informing current models of NASH should, therefore, also be considered.
Secondly, this model, as well as other NASH models, does not fully account for the fact that NASH often co-exists with other chronic, high-risk conditions such as obesity and T2D. Younossi et al. highlighted that in the US, the prevalence of NAFLD among patients with T2D was 51.8%, while the prevalence of NASH among patients with T2D was 37.3% when diagnosed via liver biopsy. Additionally, the presence of T2D amongst these patient groups has been shown to adversely affect long-term outcomes, with higher rates of clinical complications, progression to advanced fibrosis stages, and mortality associated with the combination of conditions [7]. This is evident when comparing the economic models of NAFLD developed by Younossi, inclusive and exclusive of T2D [7, 39]. The analysis including T2D utilized the previously estimated transition probabilities, while applying relative risk values of 1.78 and 1.2 to account for the increased risk of transitioning from low (F0/F1) to high fibrosis states (F2/F3) (and from high fibrosis states to cirrhosis), and from low fibrosis states to cirrhosis, respectively, when T2D was present [7]. Therefore, the inclusion of such comorbidities is a key consideration that may impact the results of cost-effectiveness analyses in this clinical area. This point is also highlighted in the systematic review and critical appraisal of NASH modeling, performed by Johansen et al. [52]. Their work presents common methodological flaws associated with economic models of NASH, some of which are also applicable to the ICER NASH model as well as to our own analysis. Notably, they highlight the fact that the treatment benefits of pharmacologic agents may extend beyond the hepatocyte, and therefore there is an unmet need for economic models of NASH to consider CV outcomes and comorbidities, such as obesity and T2D, in combination [52].
Further limitations related to the estimation of CV outcomes exist in this analysis, given that the model only estimates the CV risk based on a short-term (36 weeks) effect of resmetirom on CV risk factors using the Framingham Risk Score calculation [34]. The effect of resmetirom treatment on LDL-C is likely to continue to influence CV risk beyond the first year, where treatment effect was likely concentrated. Therefore, the prolonged effects of treatment on LDL-C and CV risk are not fully captured in this analysis. Related to this, it has been assumed that the underlying risk of CV events may be accurately predicted by the Framingham Risk Score calculation [34], alongside the impact of treatment on HDL-C, LDL-C, and triglyceride levels (as well as information on the risk ratio associated with a 1-mmol change in LDL-C levels, based on data from ICER [20]). However, there are questions surrounding this approach and the generalizability of the Framingham Risk Score amongst this population, given that the correlation between NASH and CV events is multifactorial rather than direct. Additionally, the analyses and model structure presented focused on the impact of treatment on liver fibrosis, rather than NASH resolution. As a result, the benefits associated with treatment that may impact patients downstream of the development of fibrosis were not directly captured in the model. Therefore, independently including NASH resolution, or disease activity as measured by NAS, as a clinical endpoint in the model analysis is an important consideration, and a limitation of the analysis performed by the group at ICER [20], and within our own analysis.
There were additional data limitations in this analysis that should be highlighted. Data on the proportion of patients achieving improvement in fibrosis on resmetirom, and placebo, were based on 36-week data from Loomba et al. [23]. Longer-term and more frequent follow-up data were unavailable to inform this parameter. Therefore, effectiveness data were extrapolated from 36 months to a duration of 30 years, which assumes continued effectiveness as well as a fixed rate of adherence to treatment (95%). There were also limitations in assessing quality of life due to weak evidence around utility values associated with NASH. The utility values associated with CC, DCC, and HCC were sourced from an earlier cost-effectiveness analysis [39], which identified data from a number of previous studies [40, 41, 53, 54]. However, it should be noted that the utility value for HCC was extracted from a study of patients with hepatitis C [41], while also being based on an assumption [39]. As shown in sensitivity analysis, utility values associated with both early- and late-stage disease have the potential to impact cost-effectiveness results, and therefore robust data should be used. Finally, data on the percentage of patients who remained compliant on resmetirom, and the duration of time over which patients would receive treatment, were based on assumption. These are important parameters, and future analyses of resmetirom should aim to utilize more robust data when assessing potential cost effectiveness.
Despite these limitations, there are a number of strengths to the analysis. The model follows ISPOR and NICE best-practice guidelines for performing economic evaluation [21] and is also the first economic model to explore the potential cost effectiveness of resmetirom; therefore, is valuable in informing decision making around NASH disease management in the US health care setting. The model covers the management of the condition with and without resmetirom (compared with placebo) and has a comprehensive model structure which includes all the disease-relevant health states and complications that may be experienced over a lifetime time horizon (as well as a sub-model to account for occurrence of prior CV events). The model relies on clinical and cost data primarily derived from the resmetirom phase II trial [18], the study by Loomba et al. [23], and the ICER NASH model based on an analysis of obeticholic acid [20]. The analysis has also accounted for uncertainty in the model results by performing sensitivity analysis and price threshold analysis, and considering alternative approaches to estimating CV risk, allowing for an exploration of various CV scenarios.
An updated version of this model may be utilized when further clinical data are available for resmetirom; most notably the phase III data which will focus on an F2/F3 population. The analysis may also be strengthened through use of individual-level modeling to estimate cost effectiveness (value for money) and budget impact (affordability) of resmetirom based on the clinical outcomes of individual patients and various target subgroups of NASH. This modeling approach has the potential to address heterogeneity of NASH populations, including consideration of the non-linear nature of disease progression and of rapid progressors within the cohort [3], as described by Brennan et al. [55]. Additionally, individual-level modeling has the capability of more accurately considering important parameters such as the time to costly events (i.e., LT), patient age, and key outcomes such as death [56], which can help identify the most appropriate target subgroups (e.g., F2/F3) for resmetirom to achieve higher efficacy for patients and affordability for payers. Finally, using alternative approaches to augment cost-effectiveness results to explore other patient-centered benefits should also be considered in a final value assessment of future treatments for NASH.
5 Conclusion
As an early economic evaluation, this study demonstrated the potential economic viability of resmetirom. This medication would potentially be cost effective compared with placebo for the treatment of NASH and fibrosis at a WTP threshold of US$100,000/QALY, from a US commercial payer perspective. Further clinical data will allow for a more extensive and robust analysis to be performed; potentially through use of individual-level modeling.
References
Stanfordhealthcare.org. 2021. Nonalcoholic Steatohepatitis (NASH). [online] https://stanfordhealthcare.org/medical-conditions/liver-kidneys-and-urinary-system/nonalcoholic-steatohepatitis-nash.html. Accessed 22 Feb 2022.
nhs.uk. 2022. Non-alcoholic fatty liver disease (NAFLD). [online]. https://www.nhs.uk/conditions/non-alcoholic-fatty-liver-disease/. Accessed 22 Feb 2022.
Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13(4):643–54.
Cks.nice.org.uk. 2021. Prevalence | Background information | Non-alcoholic fatty liver disease (NAFLD) | CKS | NICE. [online] https://cks.nice.org.uk/topics/non-alcoholic-fatty-liver-disease-nafld/background-information/prevalence/. Accessed 22 Feb 2022.
Garg K, Reinicke T, Garg SK. NAFLD and NASH and Diabetes. Diabetes Technology & Therapeutics. 2021;S-198-S-205. https://doi.org/10.1089/dia.2021.2515.
Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of non-alcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–33.
Younossi ZM, Tampi RP, Racila A, Qiu Y, Burns L, Younossi I, Nader F. Economic and clinical burden of nonalcoholic steatohepatitis in patients with type 2 diabetes in the U.S. Diabetes Care. 2020;43(2):283–89.
Canaby A, Kachru N, Haas JS, Meise D, Ozbay AB, Sowa JP. Healthcare resource utilization and costs among nonalcoholic fatty liver disease patients in Germany. Ann Transl Med. 2021;9(8):615.
Romero-Gomez M, Kachru N, Zamorano MA, Darba J, Shreay S. Disease severity predicts higher healthcare costs among hospitalized nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH) patients in Spain. Medicine (Baltimore). 2020;99(50): e23506.
Boursier J, Shreay S, Fabron C, et al. Hospitalization costs and risk of mortality in adults with nonalcoholic steatohepatitis: analysis of a French national hospital database. EClinicalMedicine. 2020;25: 100445.
O’Hara J, Finnegan A, Dhillon H, Ruiz-Casas L, Pedra G, Franks B, Morgan G, Hebditch V, Jönsson B, Mabhala M, Reic T, Van Thiel I, Ratziu V, Romero-Gomez M, Bugianesi E, Schattenberg JM, Anstee QM. Cost of non-alcoholic steatohepatitis in Europe and the USA: the GAIN study. JHEP Rep. 2020;2(5): 100142.
[online] https://liverfoundation.org/for-patients/about-the-liver/diseases-of-the-liver/nonalcoholic-steatohepatitis-information-center/nash-definition-prevalence/. Accessed 22 Feb 2022.
Caussy C, Reeder SB, Sirlin CB, Loomba R. Noninvasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH Trials. Hepatology. 2018;68(2):763–72.
Stine JG, Munaganuru N, Barnard A, Wang JL, Kaulback K, Argo CK, Singh S, Fowler KJ, Sirlin CB, Loomba R. Change in MRI-PDFF and histologic response in patients with nonalcoholic steatohepatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021;19(11):2274–83.
Fda.gov. 2018. Noncirrhotic nonalcoholic steatohepatitis with liver fibrosis: developing drugs for treatment guidance for industry. [online] https://www.fda.gov/media/119044/download. Accessed 14 Mar 2022.
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–57.
Madrigal Pharmaceuticals. [online] https://www.madrigalpharma.com/ourapproach/mgl3196/. Accessed 22 Feb 2022.
Harrison SA, Bashir MR, Guy CD, Zhou R, Moylan CA, Frias JP, Alkhouri N, Bansal MB, Baum S, Neuschwander-Tetri BA, Taub R, Moussa SE. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019;394(10213):2012–24.
Harrison SA, Cubberley S, Taub RA, Neff GW, Alkhouri N, Bashir M. Biomarkers, imaging and safety in resmetirom 52 week non-cirrhotic NASH phase 3 clinical trial, completed open-label arm of MAESTRO-NAFLD-1.
Institute for Clinical and Economic Review. Obeticholic acid for the treatment of nonalcoholic steatohepatitis with fibrosis-evidence report. Prepared for Midwest Comparative Effectiveness Public Advisory Council. 2021 July.
Caro JJ, Briggs AH, Siebert U, et al. Modeling good research practices—overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-1. Value Health. 2012;15(5):796–803.
Thokala P, Carlson JJ, Drummond M. HTA’d in the USA: a comparison of ICER in the United States with NICE in England and Wales. J Manag Care Spec Pharm. 2020;26(9):1162–70.
Loomba R, Guy C, Bedossa P, Taub R, Bashir M. Harrison S Magnetic resonance imaging proton density fat fraction (MRI-PDFF) to predict treatment response on NASH liver biopsy: a secondary analysis of the resmetirom randomized placebo-controlled Phase 2 clinical trial. J Hepatol. 2020;73:S56.
Loomba R. MRI-proton density fat fraction treatment response criteria in nonalcoholic steatohepatitis. Hepatology. 2021;73(3):881–3.
Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA; NASH Clinical Research Network (CRN). Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53(3):810–20.
Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multinational cohort study. Gastroenterology. 2018;155(2):443–57.
Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51(6):1972–8.
Pearson SD, Banken R, Chapman R, et al. Obeticholic Acid for the treatment of nonalcoholic steatohepatitis: comparative clinical effectiveness and value: final report. The Institute for Clinical and Economic Review (ICER). Draft Evidence Report—OCA for the treatment of NASH. 2016.
Thuluvath PJ, Hanish S, Savva Y. Waiting list mortality and transplant rates for NASH cirrhosis when compared with cryptogenic, alcoholic, or AIH cirrhosis. Transplantation. 2019;103(1):113–21.
Rustgi VK, Duff SB, Elsaid MI. Cost-effectiveness and potential value of pharmaceutical treatment of nonalcoholic fatty liver disease. J Med Econ. 2022;25(1):347–55.
Lidgren M, Hollander A, Weiland O, Jönsson B. Productivity improvements in hepatitis C treatment: impact on efficacy, cost, cost-effectiveness and quality of life. Scand J Gastroenterol. 2007;42(7):867–77.
Benyone RC, Iredale JP. Is liver fibrosis reversible? Gut. 2000;46:443–6.
Mayo Clinic. 2021. Cirrhosis—symptoms and causes. [online] https://www.mayoclinic.org/diseases-conditions/cirrhosis/symptoms-causes/syc-20351487. Accessed 21 Apr 2022.
Wannamethee SG, Shaper AG, Lennon L, Morris RW. Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med. 2005;165(22):2644–50.
Centers for Disease Control and Prevention. 2020. Current cigarette smoking among adults in the United States. [online] https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm. Accessed 22 Feb 2022.
Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–596.
Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and metaanalysis. JAMA. 2016;316(12):1289–97.
Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M. Cardiovascular risk in post-myocardial infarction patients: nationwide real world data demonstrate the importance of a long-term perspective. Eur Heart J. 2015;36(19):1163–70.
Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577–86.
Dan AA, Younossi ZM. Long-term improvement in health-related quality of life after orthotopic liver transplantation. Liver Transpl. 2008;14(10):1404–5.
Hsu PC, Federico CA, Krajden M, et al. Health utilities and psychometric quality of life in patients with early- and late-stage hepatitis C virus infection. J Gastroenterol Hepatol. 2012;27(1):149–57.
Yang LS, Shan LL, Saxena A, Morris DL. Liver transplantation: a systematic review of long-term quality of life. Liver Int. 2014;34(9):1298–313.
Sullivan PW, Ghushchyan V. Preference-Based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26(4):410–20.
Kazi DS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316(7):743–53.
O’Sullivan AK, Rubin J, Nyambose J, Kuznik A, Cohen DJ, Thompson D. Cost estimation of cardiovascular disease events in the US. Pharmacoeconomics. 2011;29(8):693–704.
Eppi.ioe.ac.uk. 2019. CCEMG - EPPI-Centre Cost Converter v.1.4. [online] https://eppi.ioe.ac.uk/costconversion/default.aspx. Accessed 3 Mar 2022.
Chirikov V, Ma I, Joshi N, Patel D, Smith A, Giambrone C, Cornelio N, Hashemi L. Cost-effectiveness of alemtuzumab in the treatment of relapsing forms of multiple sclerosis in the United States. Value Health. 2019;22(2):168–76.
Institute for Clinical and Economic Review. ICER’s Reference case for economic evaluations: principles and rationale. 2020. http://icer.org/wpcontent/uploads/2020/10/ICERReferenceCase013120.pdf. Accessed 2 Aug 2022.
Clinicaltrials.gov. 2016. Phase 2 study of MGL-3196 in patients with non-alcoholic steatohepatitis (NASH)—full text view—ClinicalTrials.gov. [online] https://clinicaltrials.gov/ct2/show/NCT02912260. Accessed 3 Mar 2022.
Younossi ZM, Ratziu V, Loomba R, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394(10215):2184–96.
Younossi ZM, Tampi R, Priyadarshini M, Nader F, Younossi IM, Racila A. Burden of illness and economic model for patients with nonalcoholic steatohepatitis in the United States. Hepatology. 2019;69(2):564–72.
Johansen P, Howard D, Bishop R, Moreno SI, Buchholtz K. Systematic literature review and critical appraisal of health economic models used in cost-effectiveness analyses in non-alcoholic steatohepatitis: potential for improvements. Pharmacoeconomics. 2020;38(5):485–97.
Younossi ZM, Boparai N, Price LL, Kiwi ML, McCormick M, Guyatt G. Health-related quality of life in chronic liver disease: the impact of type and severity of disease. AmJ Gastroenterol. 2001;96(7):2199–205.
McLernon DJ, Dillon J, Donnan PT. Health-state utilities in liver disease: a systematic review. Med Decis Making. 2008;28(4):582–92.
Brennan A, Chick SE, Davies R. A taxonomy of model structures for economic evaluation of health technologies. Health Econ. 2006;15(12):1295–310.
Who.int. 2003. [online] https://www.who.int/choice/publications/p_2003_generalised_cea.pdf. Accessed 22 Feb 2022.
Acknowledgements
Authors express their deep gratitude to clinical experts Dr Elliott Tapper and Dr Mazen Noureddin, who have reviewed all modeling assumptions. Moreover, the authors acknowledge the cooperation of Dr John Kastelein and Dr Kausik Ray who also reviewed the modeling, providing specific expert input into the cardiovascular assumptions.
Author information
Authors and Affiliations
Contributions
AA was responsible for designing and developing the economic model, performing the economic analysis, and interpreting the results. MJ designed the study and interpreted the results. EM was responsible for conducting data collection and drafting the first manuscript. JF and PR interpreted the results and critically reviewed the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Funding
This research was funded by Madrigal Pharmaceuticals, Inc. The views expressed in this publication are those of the author(s) and not necessarily those of the company.
Conflicts of interest/competing interests
MJ, EM, and AA are employees of Optimax Access Ltd., which has received research grants from Madrigal Pharmaceuticals, Inc. JF and PR are employees of Madrigal Pharmaceuticals, Inc., which funded the study.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The model used in this study was provided to the journal’s peer reviewers for their reference when reviewing the manuscript. The information reported in this manuscript is sufficient to replicate the results of the study.
Code availability
Code used to perform the economic modeling was provided to the journal’s peer reviewers for their reference when reviewing the manuscript. The information reported in this manuscript is sufficient to replicate the results of the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Javanbakht, M., Fishman, J., Moloney, E. et al. Early Cost-Effectiveness and Price Threshold Analyses of Resmetirom: An Investigational Treatment for Management of Nonalcoholic Steatohepatitis. PharmacoEconomics Open 7, 93–110 (2023). https://doi.org/10.1007/s41669-022-00370-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-022-00370-2