Abstract
Background
In the phase III PACIFIC study, durvalumab improved survival versus placebo in patients with unresectable stage III non-small-cell lung cancer (NSCLC) whose disease had not progressed after platinum-based concurrent chemoradiotherapy. The appraisal by the UK’s National Institute for Health and Care Excellence (NICE) included a cost-effectiveness analysis based on an early data readout from PACIFIC [March 2018 data cut-off (DCO); median follow-up duration 25.2 months; range 0.2–43.1]. Uncertainties regarding long-term survival outcomes with durvalumab led to some challenges in estimating the cost effectiveness of this therapy.
Objective
Here, we validate the survival extrapolations used in the original company base-case analysis by benchmarking them against updated survival data from the 4-year follow-up analysis of PACIFIC (i.e. approximately 4 years after the last patient was randomised; March 2020 DCO; median follow-up duration 34.2 months; range 0.2–64.9). Moreover, we update the original analysis with these more mature survival data to examine the consistency of key economic outputs with the original analysis.
Methods
The original analysis used a semi-Markov (state-transition) approach and was based on patients whose tumours expressed programmed cell death-ligand 1 on ≥ 1% of cells (to reflect the European licence for durvalumab). We benchmarked the survival extrapolations used in the original company base-case analysis against survival data from the 4-year follow-up of PACIFIC and updated the cost-effectiveness analysis with these more mature survival data. Early deaths avoided by the adoption of durvalumab into the UK Cancer Drugs Fund (CDF) in March 2019 were estimated using the 4-year follow-up survival data and an assumed uptake of 125 patients/year (lower estimate) and 367 patients/year (higher estimate).
Results
The original company base-case analysis had a good visual fit with the observed overall survival (OS) distribution for the durvalumab arm and accurately predicted the 48-month OS rate (predicted 55%; observed 55%); by comparison, the fit was less precise for the placebo arm, for which the analysis underestimated the 48-month OS rate (predicted 32%; observed 38%). In the updated company base-case analysis, durvalumab yielded 2.51 incremental quality-adjusted life-years (QALYs) (− 0.43 vs. the original company base-case analysis), corresponding to an incremental cost-effectiveness ratio of £22,665/QALY (+£3298 vs. the original analysis), which falls within the upper bound of NICE’s willingness-to-pay threshold (£30,000/QALY gained). We estimate that between 31 and 91 early patient deaths may have been avoided by the adoption of durvalumab into the CDF.
Conclusions
These findings reinforce the patient benefit observed with durvalumab in unresectable stage III NSCLC, support the routine use and cost effectiveness of this therapy, and demonstrate how appropriate modelling can inform the early adoption of therapies by payers to achieve patient benefit.
Plain Language Summary
Based on the results of a clinical trial, the European Medicines Agency approved durvalumab for the treatment of adults with a specific type of advanced lung cancer whose tumours cannot be removed surgically and whose disease has not progressed after chemotherapy and radiotherapy. The UK’s National Institute for Health and Care Excellence (NICE) invites companies to submit cost-effectiveness analyses to help with decision making about adopting new therapies. The company included an analysis based on early trial data that suggested durvalumab was cost effective compared with other previous treatments. As patients in the study at the time of the initial submission to NICE were only followed for approximately 2 years, the long-term survival benefit that could be achieved with durvalumab was uncertain. Therefore, NICE recommended durvalumab for use within the Cancer Drugs Fund (CDF) to allow patients to access the drug while more data were being collected. Here, we demonstrate that the original cost-effectiveness model accurately predicted the rates of long-term survival for patients receiving durvalumab and that durvalumab remains a cost-effective use of healthcare resources based on recently published data from the trial (which added approximately 2 further years of follow-up). Moreover, we estimate that adopting durvalumab into the CDF may have avoided 31–91 early patient deaths from lung cancer. These findings support NICE’s early decision to make durvalumab available within the CDF and the adoption of durvalumab for routine use within the UK national health service.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Lung cancer is the leading cause of cancer-related death worldwide (with approximately 1.8 million deaths per annum) [1], representing a considerable health and economic burden [2]. Non-small-cell lung cancer (NSCLC) accounts for at least 85% of all lung cancer diagnoses [3, 4]. Approximately 20–30% of patients with NSCLC are diagnosed with stage III (locally advanced) disease [4,5,6], many of whom are deemed unsuitable for surgical tumour resection. Historically, platinum-based chemotherapy and radiotherapy (chemoradiotherapy [CRT]) with active follow-up was the standard of care for patients with unresectable stage III NSCLC [7]. Despite the availability of this curative-intent treatment strategy [8], most patients are not cured. Ultimately, uncured patients experience disease progression, which negatively affects their health-related quality of life [9, 10]. Without a cure, patients typically die of their disease or its sequelae [9, 11]. Median progression-free survival (PFS) with concurrent CRT ranged from ~8 to 12 months, and 5-year overall survival (OS) rates ranged from 15 to 32% [7, 11,12,13], underscoring the need for novel treatments to improve outcomes in this non-metastatic disease setting, where treatment intent remains curative [8, 14].
Immune-checkpoint inhibition of the programmed cell death-1/programmed cell death-ligand 1 (PD-1/PD-L1) pathway has become a major component of oncology management for several tumour types (e.g. NSCLC and melanoma), as reflected in international treatment guidelines [15,16,17,18,19,20,21]. Until recently, this class of therapy was only available to patients with metastatic (stage IV) disease (in the NSCLC setting), many of whom may not be able to derive the full benefits of this class of therapy. The phase III PACIFIC study was the first registrational trial to demonstrate improved survival with PD-1/PD-L1 inhibition in the curative-intent NSCLC setting [22, 23]. The study assessed durvalumab (a PD-L1 checkpoint inhibitor [24]) in patients with unresectable stage III NSCLC whose disease had not progressed after platinum-based concurrent CRT (≥ 2 cycles). Patients were randomised to receive durvalumab 10 mg/kg intravenously every 2 weeks for up to 12 months or placebo, irrespective of PD-L1 tumour cell (TC) expression level or oncogenic-driver-gene mutation status. Durvalumab was associated with statistically significant and clinically meaningful improvements in the primary endpoints of OS and PFS (assessed by blinded independent central review) versus placebo in the intent-to-treat (ITT) population [22, 23]. Moreover, durvalumab had a manageable safety profile and did not detrimentally impact on patient-reported outcomes (symptoms, functioning, and global health status/quality of life) compared with placebo [22, 23, 25].
Based on an exploratory post hoc analysis from PACIFIC, the European Medicines Agency (EMA) approved durvalumab for adult patients with locally advanced unresectable NSCLC whose tumours express PD-L1 on ≥ 1% of cells and whose disease has not progressed following platinum-based CRT (approved in September 2018) [26,27,28]. Consistent with the ITT population, OS and PFS were improved with durvalumab in the PD-L1 TC ≥ 1% population (OS hazard ratio [HR] 0.53; 95% confidence interval [CI] 0.36–0.77; PFS HR 0.46; 95% CI 0.33–0.64) [26, 28]. To date, durvalumab remains the only therapy approved for patients with stage III NSCLC whose disease has not progressed following CRT [8, 14, 21].
In the UK, the National Institute for Health and Care Excellence (NICE) invites companies to submit cost-effectiveness analyses as part of the single technology appraisal (STA) process [29]. The company submission included an analysis based on the PD-L1 TC ≥ 1% population of PACIFIC (aligned with the EMA-approved indication) using data from the March 2018 data cut-off (DCO; median follow-up duration in all patients 25.2 months; range 0.2–43.1) [30], which was the most matured data available from the study at the time. The de novo base-case analysis used a state-transition modelling approach because evidence from PACIFIC indicated that the benefit of durvalumab was primarily driven by PFS [22, 23, 30], whereas post-progression survival (PPS) was similar between the durvalumab and placebo arms [30]. In the company base-case analysis, durvalumab therapy resulted in an expected gain of 2.93 quality-adjusted life-years (QALYs) compared with standard-of-care active follow-up (placebo), corresponding to a deterministic incremental cost-effectiveness ratio (ICER) of £19,366/QALY gained (after incorporating minor adjustments to correct errors identified by the NICE-commissioned evidence review group [ERG]) [30, 31]. Therefore, this ICER fell below the £20,000–30,000/QALY gained willingness-to-pay threshold that NICE applies for cost effectiveness.
During the health-technology assessment process, the ERG questioned the assumptions used in the company base-case analysis and noted that, as survival data from PACIFIC were immature at the time of the submission, any choice for modelling PFS was associated with substantial uncertainty (and different PFS model choices had a large impact on the ICER) [30, 31]. In light of the level of uncertainty, NICE recommended durvalumab for use within the Cancer Drugs Fund (CDF; a source of funding for cancer drugs in England) in March 2019 to enable patient access while longer-term survival data from the ongoing PACIFIC study were being collected [32]. A drug is recommended for use within the CDF when it is plausible that it will satisfy criteria for routine commissioning but further data collection is required to address remaining clinical uncertainty [33].
A 4-year follow-up analysis of PACIFIC was published recently (i.e. approximately 4 years after the last patient was randomised; March 2020 DCO; median follow-up duration in all patients 34.2 months; range 0.2–64.9) and demonstrated sustained OS and PFS benefit with durvalumab therapy in both the ITT and the PD-L1 TC ≥ 1% populations (PD-L1 TC ≥ 1%: OS HR 0.60; 95% CI 0.43–0.84, Kaplan–Meier [KM]-estimated median 57.4 vs. 29.6 months for durvalumab vs. placebo; PFS HR 0.49; 95% CI 0.36–0.66; median 23.9 vs. 5.6 months) [34]. These longer-term follow-up data present the opportunity to explore whether the original model submitted to NICE represented an accurate estimate of the cost effectiveness of durvalumab and could provide a valuable perspective on the use of this health economic analysis methodology to aid early decision making regarding novel anti-cancer therapies (i.e. while survival data are relatively immature and pivotal clinical trials are ongoing). This case study can also provide a perspective on the benefits of conditional reimbursement of novel anti-cancer therapies in the stage III NSCLC setting in the context of the CDF.
Here, we validate the survival extrapolations used in the original company base-case analysis by benchmarking them against updated survival data from the 4-year follow-up of PACIFIC. Moreover, we update the original analysis with these more mature survival data to examine the consistency of key economic outputs with the original analysis.
2 Methods
A de novo semi-Markov (state-transition) modelling approach was used for the analysis included in the initial company submission to NICE (based on the March 2018 DCO of PACIFIC). Full details of this model can be found in the company submission, available as part of the NICE appraisal committee papers [30]. The analysis was conducted based on the full durvalumab list price of £592 for 120 mg/£2466 for 500 mg. After incorporating minor adjustments to correct errors identified by the ERG, the company base-case analysis submitted to NICE yielded a deterministic ICER of £19,366/QALY gained [30, 31]; hereafter referred to as the ‘original company base-case analysis’. Apart from the minor corrective adjustments, the analysis reported here is consistent with that reported in the original company submission. Based on the parametric distributions fitted to the time to progression (TTP), PFS, and PPS curves, the original company base-case analysis predicted 48-month OS rates of 55 and 32% with durvalumab and placebo, respectively [30].
Here, we assessed the validity of the original company base-case analysis for modelling OS (modelled indirectly based on TTP, PFS, and PPS) and PFS by comparing the March 2018 DCO modelled survival outputs to the observed survival data from the March 2020 DCO (i.e. the 4-year update). Furthermore, we updated the original company base-case analysis with the March 2020 DCO survival data to examine the consistency of key economic outputs with the original analysis. Apart from use of more recent survival data from PACIFIC (March 2020 DCO), the modelling inputs and assumptions for the updated company base-case analysis (including costs, adverse events, subsequent therapy, and utilities) remained consistent with the original analysis. The reader is referred to the NICE STA committee papers for more details of the inputs for the model [30].
2.1 Patient Population
Modelling was based on the PD-L1 TC ≥ 1% population from PACIFIC to reflect the EMA-approved indication for durvalumab [28]. As described elsewhere [26], this subpopulation represented 42% of the full ITT population. Baseline clinical characteristics were well balanced between the durvalumab and placebo arms within this subpopulation (and were consistent with the ITT population) [26]; this view was reflected in the comments from the NICE-commissioned ERG [30].
2.2 Cost-Effectiveness Model Structure
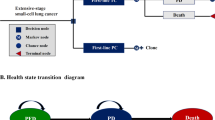
A three-state semi-Markov (state-transition) model was used for the base-case analysis (Table 1) [30]. Modelling was performed from a UK health-payer perspective and over a lifetime (40-year) time horizon. All costs, life-years (LYs), and QALYs were discounted at a rate of 3.5% per year. For the model structure, the three health states were progression free, progressed disease, and death (Fig. 1). PFS, TTP, and PPS data from PACIFIC were used to generate probabilities that patients remained in the same health state or transitioned to a subsequent health state. As described previously, parametric distributions were fitted to observed (KM) data from PACIFIC to extrapolate survival outcomes beyond the study follow-up period [30]. The treatment benefit cut-off point (i.e. the treatment effect duration) for durvalumab was set at 10 years (i.e. at this point, the model assumes that the hazards for progression and death become identical between the durvalumab and placebo arms).
Health-state structure used in the economic model. The health-state structure was published previously as part of the UK National Institute for Care and Excellence single technology appraisal committee papers [30]. PFS progression-free survival, PPS post-progression survival, TTP time to progression
2.3 Validation of the Original Company Base-Case Analysis for Modelling Longer-Term (4-Year) Survival Outcomes
To assess the accuracy of the original company base-case analysis (based on the March 2018 DCO of PACIFIC) for predicting longer-term survival outcomes, the modelled OS and PFS distributions from this analysis were compared with the observed OS and PFS (KM) data from the March 2020 DCO. We evaluated the harmony between the predicted and observed point estimates for the 48-month OS and PFS rates and the visual goodness of fit of the modelled survival distributions to the observed data.
2.4 Examination of the Consistency of the Economic Outputs from the Original and Updated Company Base-Case Analyses
We updated the original company base-case analysis with the March 2020 DCO survival data (PD-L1 TC ≥ 1% population) and examined the consistency of results for QALYs, LYs, costs, and ICERs obtained in the original and updated analyses.
Consistent with the approach used for the original company base-case analysis (as reported in the NICE STA committee papers [30]), parametric survival distributions were fitted to patient-level data from the March 2020 DCO dataset to identify the best fitting models for estimating TTP, PFS, and PPS in the updated company base-case analysis (PPS data were pooled across the study arms; thus, the model assumes that PPS is equal for both arms [30]). The choice of parametric distributions for estimating TTP and PFS in the updated company base-case analysis, based on Akaike and Bayesian information criteria (AIC and BIC, respectively), was consistent with the original analysis (generalised gamma) (Table 1 in the electronic supplementary material [ESM]). Based on AIC, the log-logistic distribution had a better statistical fit for the March 2020 DCO PPS data (whereas the exponential distribution had the best fit for the March 2018 DCO and so was used to model PPS in the original analysis [30]) (Table 2 in the ESM). As there was only a 1.7-point difference in AIC score between the log-logistic and exponential distributions for the March 2020 DCO, the exponential distribution (which still had the best statistical fit according to BIC) was retained for modelling PPS in the updated analysis for consistency with the original company base-case analysis; the log-logistic distribution was explored in a sensitivity analysis.
Results for QALYs, LYs, costs, and ICERs from the updated analysis were compared with the values obtained from the original company base-case analysis. As mentioned, apart from the use of updated survival distributions (based on the March 2020 DCO), all other modelling inputs and assumptions used for the updated analysis remained consistent with the original analysis. We also assessed the validity of the modelled OS and PFS distributions from the updated analyses by comparing them with the observed (KM) data from the March 2020 DCO for visual goodness of fit.
2.5 Sensitivity Analyses
Specific sensitivity analyses were carried out to explore the impact of the following variables on the ICER (based on the March 2020 DCO survival data). These included (1) using a log-logistic distribution for modelling PPS (to account for changes in the AIC/BIC with the more recent survival data) and (2) setting the treatment effect duration for durvalumab at 5 years (given that this is an ongoing area of uncertainty).
2.6 Assessment of the Impact of the Early Availability of Durvalumab Within the Cancer Drugs Fund
As NICE approved durvalumab for reimbursement within the CDF to allow early managed access to this novel agent (approximately 2 years before median OS maturing for the durvalumab arm in PACIFIC [34]), we sought to estimate the impact of this decision in terms of the number of early patient deaths avoided in the UK within the first 2 years of durvalumab being available. We determined this by calculating the difference in the proportions of patients alive at the 2-year landmark in the durvalumab and placebo arms of PACIFIC (PD-L1 TC ≥ 1% population; March 2020 DCO) and using the resulting figure to calculate the number of patients for whom early death was avoided based on an uptake of 125 patients/year (as estimated by NICE [35]) and 367 patients/year (estimated using data from the National Lung Cancer Audit and reported in the committee papers [30]). The modelling assumed that the patients who started treatment were equally distributed over the 2-year period on a monthly basis (with survival based on OS KM data from the March 2020 DCO).
3 Results
3.1 Validation of the Modelled Overall Survival and Progression-Free Survival Outcomes from the Original Company Base-Case Analysis (Based on 2-Year Follow-Up Data) with Observed 4-Year Follow-Up Data from PACIFIC
The modelled OS and PFS distributions from the original company base-case analysis (March 2018 DCO) are shown benchmarked with the observed (KM) data from the 4-year follow-up of PACIFIC (March 2020 DCO; PD-L1 TC ≥ 1% population) (Fig. 2). PFS and OS distributions from the updated company base-case analysis (which used the March 2020 DCO to model survival) are also shown for completeness and discussed further in the following.
Overlay of the modelled (A) OS and (B) PFS distributions from the original and updated company base-case analyses with the observed 4-year survival data from the PACIFIC study (PD-L1 tumour cell ≥ 1% population). Modelled OS is derived indirectly using time to progression, PFS, and post-progression survival. The original company base-case analysis was based on survival data from the March 2018 data cut-off of the PACIFIC study [26, 30], and the updated company base-case analysis was based on 4-year follow-up survival data from the March 2020 data cut-off of the PACIFIC study [34]. Data for the OS and PFS Kaplan–Meier distributions (March 2020 data cut-off; illustrated by the dashed lines) were reported in a separate publication [34]. OS overall survival, PD-L1 programmed cell death-ligand 1, PFS progression-free survival
As of 22 March 2020 (approximately 4 years after the last patient was randomised), the median follow-up duration in all patients was 40.0 months (range 0.2–64.5) for the durvalumab arm and 26.4 months (range 0.3–64.9) for the placebo arm. The modelled OS distribution from the original company base-case analysis had a good visual fit to the updated OS data for the durvalumab arm (Fig. 2A) and accurately predicted the 48-month OS rate for durvalumab (predicted 55%; observed 55%; difference 0%) (Table 2). The fit was less precise for the placebo arm (Fig. 2A), and the original model under-predicted the 48-month OS rate for placebo (predicted 32%; observed 38%; difference −7%); this was attributed to a further flattening of the tail of the OS KM distribution for the placebo arm at the March 2020 DCO relative to the March 2018 DCO (and thus a greater proportion of long-term survivors than had been expected based on the March 2018 DCO) [23, 26, 30, 34].
The modelled PFS distribution from the original company base-case analysis had a good visual fit to the updated PFS data for both the durvalumab and the placebo arms (Fig. 2B), although the model slightly over-predicted the 48-month PFS rate for durvalumab (difference + 4%) and slightly under-predicted the rate for placebo (difference − 4%).
3.2 Updated Company Base-Case Analysis (Based on 4-Year Follow-Up Data from PACIFIC)
The modelled OS distributions from the updated company base-case analysis (March 2020 DCO) had a good visual fit to the observed OS KM data for the durvalumab arm from the 4-year follow-up of PACIFIC (March 2020 DCO; PD-L1 TC ≥ 1% population); the fit was less precise for the placebo arm (as observed in the original company base-case analysis) (Fig. 2A). Meanwhile, the modelled PFS distributions from the updated analysis had a good visual fit to the observed PFS KM data for both the durvalumab and the placebo arms up to 48 months. After 48 months, censoring substantially reduced the numbers of patients at risk, meaning there is greater uncertainty in the KM curves beyond 48 months, resulting in modelled curves that do not follow the KM curves as closely beyond this timepoint (Fig. 2B). The outputs from the updated analysis (March 2020 DCO) approximated with those from the original analysis (March 2018 DCO) (Table 3): durvalumab yielded 2.51 (discounted) incremental QALYs (− 0.43 vs. the original analysis) and 3.07 (discounted) incremental LYs (− 0.53) compared with standard-of-care active follow-up (placebo), corresponding to an ICER of £22,665/QALY gained (+ £3298). Therefore, durvalumab therapy remains a cost-effective use of national health service (NHS) resources, falling within the upper bound of the NICE willingness-to-pay threshold (£20,000–30,000). Sensitivity analyses demonstrated that the use of a log-logistic distribution for modelling PPS (£23,762) and setting the treatment effect duration for durvalumab at 5 years (£27,612) did not have a substantial impact on the ICER (March 2020 DCO).
3.3 Impact of Durvalumab Availability in the Cancer Drugs Fund
Based on 2-year landmark OS data from PACIFIC (based on the March 2020 DCO), the early availability of durvalumab in the UK (within the CDF) was estimated to have avoided between 31 and 91 early deaths in the first 2 years following its introduction, assuming an uptake of between 125 patients/year (per the NICE estimate [35]) and 367 patients/year (as described in the company submission [30]), respectively (Table 4).
4 Discussion
Validating the original cost-effectiveness analysis submitted to NICE by benchmarking it against more mature (4-year) survival data from PACIFIC supports the modelling approach used for the company base-case analysis and provides a valuable case study on how the cost effectiveness of novel anti-cancer therapies can be predicted reliably based on relatively immature survival data. Moreover, updating the cost-effectiveness analysis with these more mature survival data provides the opportunity to measure the long-term benefit of durvalumab more accurately.
Our findings demonstrate that the semi-Markov approach used for the original company base-case analysis predicted OS with durvalumab therapy with a high degree of precision, as demonstrated by the good visual fit between the modelled (March 2018 DCO) and observed (March 2020 DCO) OS data and the accurate prediction of the 48-month OS rate for the durvalumab arm of PACIFIC (predicted 55%; observed 55%). Meanwhile, the analysis was less accurate at predicting the 48-month OS rate for the placebo arm (predicted 32%; observed 38%). The less accurate fit of the model for the placebo arm appeared to be due to further flattening of the OS KM distribution for this arm at the March 2020 DCO compared with the March 2018 DCO [23, 26, 30, 34]. This led to under-prediction of the 48-month OS rate for the placebo arm by the original analysis. Despite this, the ICERs yielded by the original (March 2018 DCO) and updated (March 2020 DCO) company base-case analyses were similar (difference £3298), indicating that the original analysis provided a good estimate of the cost effectiveness of durvalumab therapy based on relatively immature survival data. Taken together, these findings validate the approach used in the original company base-case analysis and suggest that the underlying assumptions of the model were robust.
Other validation studies have assessed the accuracy of extrapolated OS data applied in oncology pricing and reimbursement submissions [36,37,38]. One study, which summarised 11 NICE submissions for cancer immunotherapies, found that the initial extrapolation tended to underestimate OS by 0.4–2.7% depending on the assessment method used; the authors concluded that the initial extrapolations were appropriate for health-technology assessment decisions [38]. The current study provides another example of the high level of accuracy that can be achieved when extrapolating OS with immunotherapy.
We cannot definitively explain the better-than-expected 48-month OS rate in the placebo arm at the March 2020 DCO; however, it could be related to the longer duration of follow-up at the March 2020 DCO among patients who received subsequent immunotherapy (up to additional 2 years vs. the March 2018 DCO). Indeed, 31% of patients in the placebo arm (and 12% in the durvalumab arm) had received subsequent immunotherapy by the March 2020 DCO (PD-L1 TC ≥ 1% population). Although most of these patients had commenced subsequent immunotherapy by the March 2018 DCO [30], the longer duration of follow-up at the latter DCO possibly allowed sufficient time for the OS benefit associated with immunotherapy in later treatment lines to become apparent.
Durvalumab is associated with considerable benefit in patients with unresectable stage III NSCLC, who are characterised by a substantial disease burden and, historically, a poor prognosis (despite treatment with curative intent). In the updated company base-case analysis, based on survival data from the 4-year follow-up of PACIFIC (March 2020 DCO), durvalumab therapy was associated with a gain of an additional 2.51 (discounted) QALYs versus standard-of-care active follow-up. This provides validation of the considerable patient benefit predicted by the original company base-case analysis (March 2018 DCO; 2.93 QALYs gained). Moreover, this level of QALY gain is considerably higher than that observed with immunotherapies currently approved by NICE in the advanced/metastatic NSCLC setting [39,40,41,42,43]. Durvalumab remains a cost-effective use of NHS resources at the list price of £592 for 120 mg/£2466 for 500 mg: the ICER was estimated as £22,665/QALY gained, which falls within the higher bound of the willingness-to-pay threshold that NICE applies for cost effectiveness (£30,000). This supports the adoption of durvalumab in routine clinical practice. An important caveat is that the updated cost-effectiveness analysis remains dependent on extrapolations to estimate longer-term survival (beyond 4 years).
Numerous cost-effectiveness analyses have been published based on both the ITT and the PD-L1 TC ≥ 1% PACIFIC populations. These analyses report a lower range of QALY gains with durvalumab therapy (0.24–1.32 for the PD-L1 TC ≥ 1% population) compared with the original company base-case analysis (2.93) (Table 3 in the ESM) [30, 31, 44,45,46,47,48]; this could be explained, in part, by the use of different modelling approaches, decision problems, and/or assumptions regarding long-term survival benefit. The recently published data from PACIFIC (March 2020 DCO) demonstrated a gain in median OS of 27.8 months with durvalumab therapy versus placebo and a sustained OS and PFS benefit with durvalumab at 4 years (PD-L1 TC ≥ 1% population) [34]; the 48-month OS rates were 55 and 38% with durvalumab and placebo, respectively. This brings into question whether the other models in the literature that report lower QALY gains for durvalumab therapy reflect the most recent evidence of clinical efficacy from PACIFIC and accurately represent patient and health-system benefits.
The aim of the current analysis was limited to validating the original company base-case analysis submitted to NICE by benchmarking it against, and updating it with, more mature survival data from PACIFIC. The validity of alternative model structures was not explored, nor were other parameters updated (e.g. costs). Moreover, complex hazard functions were not considered (e.g. methods that incorporate splines or fractional polynomials) [49]. Further analyses could be undertaken to assess the impact of key modelling choices on the estimated QALYs, LYs, costs, and ICERs.
At the time of the original company submission to NICE, the ERG expressed concern that the choice of a generalised-gamma distribution to model PFS in the company base-case analysis caused a potential overestimation of the long-term survival benefit that could be achieved with durvalumab therapy and suggested that using a log-normal distribution for the durvalumab arm may be a more appropriate choice for decision making [30, 31]. However, the ERG acknowledged that any choice for modelling PFS was associated with substantial uncertainty as survival data from PACIFIC were immature at the time of submission. The findings of the current analysis suggest that the use of a generalised-gamma distribution provided a plausible estimate of benefit with durvalumab (when benchmarked with more mature survival data from the 4-year follow-up of PACIFIC).
The ERG expressed a further concern that setting the treatment effect duration for durvalumab at 10 years was potentially too long and suggested a more conservative duration of 3–5 years [30, 31]. The 4-year follow-up data from PACIFIC demonstrated that OS and PFS benefit with durvalumab was sustained for at least 4 years [34], indicating that a treatment effect duration of less than this may be too conservative. Of note, setting the treatment effect duration at 5 years did not have a substantial impact on the ICER (£27,612) in the current analysis. However, we feel that 5 years is a highly conservative assumption since longer-term benefit (e.g. 10 years) is plausible in a curative-intent setting. Any future updates from the PACIFIC study may provide the opportunity to further explore uncertainties regarding the treatment effect duration.
While the original company base-case analysis demonstrated good internal validity (by accurately predicting OS for the durvalumab arm of PACIFIC from TTP, PFS, and PPS distributions), it is only one study, and it cannot be concluded that all models will be this accurate or that it is the most appropriate modelling approach for other similar decision problems. Nevertheless, the ability of the modelling approach to robustly predict long-term benefit allows payer organisations to have confidence in approving novel therapies with immature data in areas of high unmet need (in the absence of more mature data, while pivotal clinical trials are ongoing). This is supported by our finding that NICE’s decision to allow early managed access to durvalumab through the CDF may have prevented between 31 and 91 early patient deaths in the first 2 years following its introduction.
5 Conclusion
By validating the outcomes of the original cost-effectiveness analysis, this research reinforces the considerable patient benefit observed with durvalumab and supports the use of this cost-effective therapy with a higher degree of certainty (given the use of more mature survival data to update the model). It also provides an informative case study on cost-effectiveness analyses in the unresectable stage III NSCLC setting from both methodological and policy perspectives.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.Erratum.In:CACancerJClin.2020;70(4):313 (PMID: 30207593).
Wood R, Taylor-Stokes G. Cost burden associated with advanced non-small cell lung cancer in Europe and influence of disease stage. BMC Cancer. 2019;19(1):214. https://doi.org/10.1186/s12885-019-5428-4.PMID:30849964;PMCID:PMC6408828.
Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016;5(3):288–300. https://doi.org/10.21037/tlcr.2016.06.07.PMID:27413711;PMCID:PMC4931124.
Royal College of Physicians. NLCA annual report 2020 (for the 2018 audit period). 2020. https://www.rcplondon.ac.uk/projects/outputs/annual-report-published-2020. Accessed 05 Feb 2021.
Walters S, Maringe C, Coleman MP, Peake MD, Butler J, Young N, et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004–2007. Thorax. 2013;68(6):551–64. https://doi.org/10.1136/thoraxjnl-2012-202297 (PMID: 23399908).
Chen VW, Ruiz BA, Hsieh MC, Wu XC, Ries LA, Lewis DR. Analysis of stage and clinical/prognostic factors for lung cancer from SEER registries: AJCC staging and collaborative stage data collection system. Cancer. 2014;120(Suppl 23):3781–92. https://doi.org/10.1002/cncr.29045 (PMID:25412390;PMCID:PMC4239667).
Yoon SM, Shaikh T, Hallman M. Therapeutic management options for stage III non-small cell lung cancer. World J Clin Oncol. 2017;8(1):1–20. https://doi.org/10.5306/wjco.v8.i1.1.PMID:28246582;PMCID:PMC5309711.
Huber RM, De Ruysscher D, Hoffmann H, Reu S, Tufman A. Interdisciplinary multimodality management of stage III nonsmall cell lung cancer. Eur Respir Rev. 2019;28(152):190024. https://doi.org/10.1183/16000617.0024-2019 (PMID: 31285288).
Ryan KJ, Skinner KE, Fernandes AW, Punekar RS, Pavilack M, Walker MS, et al. Real-world outcomes in patients with unresected stage III non-small cell lung cancer. Med Oncol. 2019;36(3):24. https://doi.org/10.1007/s12032-019-1249-1.
Polanski J, Jankowska-Polanska B, Rosinczuk J, Chabowski M, Szymanska-Chabowska A. Quality of life of patients with lung cancer. Onco Targets Ther. 2016;9:1023–8. https://doi.org/10.2147/OTT.S100685 (PMID:27013895;PMCID:PMC4778772).
Aupérin A, Le Péchoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(13):2181–90. https://doi.org/10.1200/JCO.2009.26.2543 (PMID: 20351327).
Hansen RN, Zhang Y, Seal B, Ryan K, Yong C, Darilay A, et al. Long-term survival trends in patients with unresectable stage III non-small cell lung cancer receiving chemotherapy and radiation therapy: a SEER cancer registry analysis. BMC Cancer. 2020;20(1):276. https://doi.org/10.1186/s12885-020-06734-3 (PMID:32248816;PMCID:PMC7132866).
Bradley JD, Hu C, Komaki RR, Masters GA, Blumenschein GR, Schild SE, et al. Long-term results of NRG Oncology RTOG 0617: Standard-versus high-dose chemoradiotherapy with or without cetuximab for unresectable stage III non-small-cell lung cancer. J Clin Oncol. 2020;38(7):706–14. https://doi.org/10.1200/JCO.19.01162 (PMID:31841363;PMCID:PMC7048161).
Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Suppl 4):iv1–21. https://doi.org/10.1093/annonc/mdx222.
Hanna NH, Schneider BJ, Temin S, Baker S Jr, Brahmer J, Ellis PM, et al. Therapy for stage IV non-small-cell lung cancer without driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol. 2020;38(14):1608–32. https://doi.org/10.1200/JCO.19.03022 (PMID: 31990617).
Seth R, Messersmith H, Kaur V, Kirkwood JM, Kudchadkar R, McQuade JL, et al. Systemic therapy for melanoma: ASCO guideline. J Clin Oncol. 2020;38(33):3947–70. https://doi.org/10.1200/JCO.20.00198 (PMID: 32228358).
Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):192–237. https://doi.org/10.1093/annonc/mdy275 (Erratum in: Ann Oncol. 2019;30(5):863-870. PMID: 30285222.).
Wu YL, Planchard D, Lu S, Sun H, Yamamoto N, Kim DW, et al. Pan-Asian adapted Clinical Practice Guidelines for the management of patients with metastatic non-small-cell lung cancer: a CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30(2):171–210. https://doi.org/10.1093/annonc/mdy554 (PMID: 30596843).
Park K, Vansteenkiste J, Lee KH, Pentheroudakis G, Zhou C, Prabhash K, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with locally-advanced unresectable non-small-cell lung cancer: a KSMO-ESMO initiative endorsed by CSCO, ISMPO, JSMO, MOS, SSO and TOS. Ann Oncol. 2020;31(2):191–201. https://doi.org/10.1016/j.annonc.2019.10.026 (PMID: 31959336).
Keilholz U, Ascierto PA, Dummer R, Robert C, Lorigan P, van Akkooi A, et al. ESMO consensus conference recommendations on the management of metastatic melanoma: under the auspices of the ESMO Guidelines Committee. Ann Oncol. 2020;31(11):1435–48. https://doi.org/10.1016/j.annonc.2020.07.004 (PMID: 32763453).
Pentheroudakis G; ESMO Guidelines Committee. Recent eUpdate to the ESMO Clinical Practice Guidelines on early and locally advanced non-small-cell lung cancer (NSCLC). Ann Oncol. 2020;31(9):1265–6. https://doi.org/10.1016/j.annonc.2020.05.023 (PMID: 32502714).
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–29. https://doi.org/10.1056/NEJMoa1709937 (PMID: 28885881).
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–50. https://doi.org/10.1056/NEJMoa1809697 (PMID: 30280658).
Stewart R, Morrow M, Hammond SA, Mulgrew K, Marcus D, Poon E, et al. Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol Res. 2015;3(9):1052–62. https://doi.org/10.1158/2326-6066.CIR-14-0191 (PMID: 25943534).
Hui R, Özgüroğlu M, Villegas A, Daniel D, Vicente D, Murakami S, et al. Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): a randomised, controlled, phase 3 study. Lancet Oncol. 2019;20(12):1670–80. https://doi.org/10.1016/S1470-2045(19)30519-4 (PMID: 31601496).
Paz-Ares L, Spira A, Raben D, Planchard D, Cho BC, Özgüroğlu M, et al. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann Oncol. 2020;31(6):798–806. https://doi.org/10.1016/j.annonc.2020.03.287 (PMID: 32209338).
Peters S, Dafni U, Boyer M, De Ruysscher D, Faivre-Finn C, Felip E, et al. Position of a panel of international lung cancer experts on the approval decision for use of durvalumab in stage III non-small-cell lung cancer (NSCLC) by the Committee for Medicinal Products for Human Use (CHMP). Ann Oncol. 2019;30(2):161–5. https://doi.org/10.1093/annonc/mdy553 (PMID: 30624547).
European Medicines Agency (EMA). Imfinzi (durvalumab) Product Information. 2020. https://www.ema.europa.eu/en/documents/product-information/imfizi-epar-product-information_en.pdf. Accessed 05 Feb 2021).
National Institute for Health and Care Excellence. Single technology appraisal: User guide for company evidence submission template [PMG24]. 2015. https://www.nice.org.uk/process/pmg24/chapter/instructions-for-companies. Accessed 05 Feb 2021.
National Institute for Health and Care Excellence. Durvalumab for maintenance treatment of unresectable non-small-cell lung cancer after platinum-based chemoradiation [ID1175]: Committee Papers. 2019. https://www.nice.org.uk/guidance/ta578/evidence/committee-papers-pdf-6777578413. Accessed 05 Feb 2021.
Witlox WJA, van Asselt ADI, Wolff R, Armstrong N, Worthy G, Chalker A, et al. Durvalumab for the treatment of locally advanced, unresectable, stage III non-small cell lung cancer: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2020;38(4):317–24. https://doi.org/10.1007/s40273-019-00870-w.PMID:31814080;PMCID:PMC7080309.
National Institute for Health and Care Excellence. Durvalumab for treating locally advanced unresectable non-small-cell lung cancer after platinum-based chemoradiation: Final appraisal document. 2019. https://www.nice.org.uk/guidance/ta578/documents/final-appraisal-determination-document. Accessed 05 Feb 2021.
National Health Service England. Appraisal and Funding of Cancer Drugs from July 2016 (including the new Cancer Drugs Fund)—a new deal for patients, taxpayers and industry. 2016. https://www.england.nhs.uk/wp-content/uploads/2013/04/cdf-sop.pdf. Accessed 23 Jun 2021.
Faivre-Finn C, Vicente D, Kurata T, Planchard D, Paz-Ares L, Vansteenkiste JF, et al. Brief report: four-year survival with durvalumab after chemoradiotherapy in Stage III NSCLC—an update from the PACIFIC trial. J Thorac Oncol. 2021. https://doi.org/10.1016/j.jtho.2020.12.015.
National Institute for Health and Care Excellence. Durvalumab for treating locally advanced unresectable non-small-cell lung cancer after platinum-based chemoradiation [TA578]: resource impact statement. 2019. https://www.nice.org.uk/guidance/ta578/resources/resource-impact-statement-pdf-8783694593989. Accessed 05 Feb 2021.
Bullement A, Latimer NR, Bell GH. Survival extrapolation in cancer immunotherapy: a validation-based case study. Value Health. 2019;22(3):276–83. https://doi.org/10.1016/j.jval.2018.10.007.PMID:30832965.L.
Smare C, Lakhdari K, Doan J, Posnett J, Johal S. Evaluating partitioned survival and Markov decision-analytic modeling approaches for use in cost-effectiveness analysis: Estimating and comparing survival outcomes. Pharmacoeconomics. 2020;38(1):97–108. https://doi.org/10.1007/s40273-019-00845-x (PMID:31741315;PMCID:PMC7081655).
Bullement A, Meng Y, Cooper M, Lee D, Harding TL, O’Regan C, et al. A review and validation of overall survival extrapolation in health technology assessments of cancer immunotherapy by the National Institute for Health and Care Excellence: how did the initial best estimate compare to trial data subsequently made available? J Med Econ. 2019;22(3):205–14. https://doi.org/10.1080/13696998.2018.1547303 (PMID: 30422080).
National Institute for Health and Care Excellence. Pembrolizumab for treating PD-L1-positive non-small-cell lung cancer after chemotherapy [TA428]. 2017. (Updated: 12 September 2017). https://www.nice.org.uk/guidance/TA428. Accessed 05 Feb 2021.
National Institute for Health and Care Excellence. Nivolumab for advanced squamous non-small-cell lung cancer after chemotherapy [TA655]. 2020. https://www.nice.org.uk/guidance/ta655. Accessed 05 Feb 2021.
National Institute for Health and Care Excellence. Pembrolizumab for untreated PD-L1-positive metastatic non-small-cell lung cancer [TA531]. 2018. https://www.nice.org.uk/guidance/ta531. Accessed 05 Feb 2021.
National Institute for Health and Care Excellence. Nivolumab for previously treated non-squamous non-small-cell lung cancer [TA484]. 2017. https://www.nice.org.uk/guidance/ta484. Accessed 05 Feb 2021.
National Institute for Health and Care Excellence. Atezolizumab for treating locally advanced or metastatic non-small-cell lung cancer after chemotherapy [TA520]. 2018. https://www.nice.org.uk/guidance/ta520. Accessed 05 Feb 2021.
Criss SD, Mooradian MJ, Sheehan DF, Zubiri L, Lumish MA, Gainor JF, et al. Cost-effectiveness and budgetary consequence analysis of durvalumab consolidation therapy vs no consolidation therapy after chemoradiotherapy in stage III non-small cell lung cancer in the context of the US health care system. JAMA Oncol. 2019;5(3):358–65. https://doi.org/10.1001/jamaoncol.2018.5449 (PMID:30543349;PMCID:PMC6439842).
Han J, Tian K, Yang J, Gong Y. Durvalumab vs placebo consolidation therapy after chemoradiotherapy in stage III non-small-cell lung cancer: an updated PACIFIC trial-based cost-effectiveness analysis. Lung Cancer. 2020;146:42–9. https://doi.org/10.1016/j.lungcan.2020.05.011 (PMID: 32512272).
Panje CM, Lupatsch JE, Barbier M, Pardo E, Lorez M, Dedes KJ, et al. A cost-effectiveness analysis of consolidation immunotherapy with durvalumab in stage III NSCLC responding to definitive radiochemotherapy in Switzerland. Ann Oncol. 2020;31(4):501–6. https://doi.org/10.1016/j.annonc.2020.01.007 (PMID: 32107097).
Armeni P, Borsoi L, Fornaro G, Jommi C, Grossi F, Costa F. Cost-effectiveness and net monetary benefit of durvalumab consolidation therapy versus no consolidation therapy after chemoradiotherapy in stage III non-small cell lung cancer in the Italian National Health Service. Clin Ther. 2020;42(5):830–47. https://doi.org/10.1016/j.clinthera.2020.03.012 (PMID: 32354495).
Mehra R, Yong C, Seal B, van Keep M, Raad A, Zhang Y. Cost-Effectiveness of durvalumab after chemoradiotherapy in unresectable stage III NSCLC: a US healthcare perspective. J Natl Compr Canc Netw. 2021;19(2):153–62. https://doi.org/10.6004/jnccn.2020.7621 (PMID: 33545688).
Rutherford MJ, Lambert PC, Sweeting MJ, Pennington B, Crowther MJ, Abrams KR, Latimer NR. NICE DSU TECHNICAL SUPPORT DOCUMENT 21: flexible methods for survival analysis .2020. http://nicedsu.org.uk/wp-content/uploads/2020/11/NICE-DSU-Flex-Surv-TSD-21_Final_alt_text.pdf. Accessed: 05 Feb 2021.
NICE: Guide to the methods of technology appraisal 2013. 2013. https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781. Accessed 05 Feb 2021.
Acknowledgements
Anna Quinton, Chris Livings, Gbenga Kazeem, Annie Darilay, James Ryan, Bengt Liljas, Ned Pethick, Helen Tipping, and Dawn Lee contributed to the original company submission to NICE and/or provided support for and/or critical review of this manuscript. Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Aaron Korpal, PhD, of Ashfield MedComms (Manchester, UK), an Ashfield Health company, and was funded by AstraZeneca.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The PACIFIC study (NCT02125461) was funded by AstraZeneca.
Conflict of interest
Will Dunlop, Ignacio Diaz Perez, Mario JNM Ouwens, Tina Sarbajna, and Yiduo Zhang report employment by AstraZeneca and stock ownership in AstraZeneca. Marjolijn van Keep and Peter Elroy report employment by BresMed (under contract with AstraZeneca). Alastair Greystoke has received honoraria, speaker’s fees, and research funding from AstraZeneca.
Availability of data and materials
Details of the original economic model submitted to NICE can be found in the committee papers associated with the STA for durvalumab [ID1175] [30]. The 4-year update of clinical outcomes from the PACIFIC study is published in the Journal of Thoracic Oncology [34], and clinical outcomes for the subpopulation of patients whose tumours express PD-L1 on ≥ 1% of tumour cells are included in the online data supplement accompanying that publication. AstraZeneca’s data-sharing policy is described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Code availability
A detailed overview of the model structure and inputs has been published as in the original NICE submission (available as part of the NICE appraisal committee papers [ID1175] [30]); however, the coded model itself has not been published.
Author contributions
MvK, YZ, MJNMO, TS, PE, and AG contributed to the original company STA submission to the National Institute for Health and Care Excellence (NICE; ID1175). WD, MvK, PE, YZ, MJNMO, and TS were involved in the conception and design of the current analysis. MvK, PE, WD, MJNMO, and YZ contributed to data analysis. IDP and AG contributed to clinical interpretation of the data. A medical writer under the supervision of WD wrote the manuscript. WD, MvK, TS, AG, IDP, MJNMO, YZ, and PE reviewed the manuscript, made substantial contributions to the content, and approved the final draft.
Ethics approval
This cost-effectiveness analysis was based on data from the PACIFIC study, which was conducted in accordance with ethical principles that have their origin in the Declaration of Helsinki and are consistent with the International Council on Harmonisation guidelines for good clinical practice.
Consent to participate
All patients provided written informed consent for participation in the PACIFIC study.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Dunlop, W., van Keep, M., Elroy, P. et al. Cost Effectiveness of Durvalumab in Unresectable Stage III NSCLC: 4-Year Survival Update and Model Validation from a UK Healthcare Perspective. PharmacoEconomics Open 6, 241–252 (2022). https://doi.org/10.1007/s41669-021-00301-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-021-00301-7