Abstract
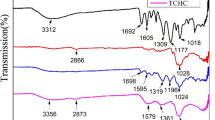
The current study describes the application of a new extraction method for efficient uranium adsorption via cost-effective hydrazine-impregnated activated carbon. Various experimental parameters such as time, adsorbent weight, temperature (°C), and uranium concentration were thoroughly investigated. The synthesized adsorbent was characterized via X-ray diffraction, Fourier transformation infrared spectroscopy (FT-IR), scanning electron microscopy, and thermogravimetric analysis. The results showed 86% uranium extraction under optimized conditions (20% P2O5 at 25 °C, 120 min). The obtained findings fit well with thermodynamic and isothermal (Langmuir and Freundlich isotherms) models and pseudo second-order kinetics. In thermodynamic studies, the negative sign of (∆G°) specified the spontaneity of process, the negative sign of (∆H°) revealed endothermicity, and the positive sign of (∆S°) showed high randomness after adsorption.









Similar content being viewed by others
References
P. Yang, Q. Liu, J. Liu et al., Highly efficient immobilization of uranium (VI) from aqueous solution by phosphonate-functionalized dendritic fibrous nanosilica (DFNS). J. Hazard. Mater. 363, 248–257 (2019). https://doi.org/10.1016/j.jhazmat.2018.09.062
F. Hameed, Hazardous effects of cadmium contaminated water on biological characteristics of fish; a review. RHAZES: Green Appl. Chem. 6, 1–10 (2019)
R. Sanghani, Novel technique for purification of fertilizer phosphoric acid with simultaneous uranium extraction. Procedia Eng. 83, 225–232 (2014). https://doi.org/10.1016/j.proeng.2014.09.042
W. Zou, L. Zhao, R. Han, Removal of uranium (VI) by fixed bed ion-exchange column using natural zeolite coated with manganese oxide. Chin. J. Chem. Eng. 17(4), 585–593 (2009). https://doi.org/10.1016/s1004-9541(08)60248-7
J. Li, L. Zhang, J. Peng et al., Removal of uranium from uranium plant wastewater using zero-valent iron in an ultrasonic field. Nucl. Eng. Technol. 48(3), 744–750 (2016). https://doi.org/10.1016/j.net.2016.01.021
R. Han, W. Zou, Y. Wang et al., Removal of uranium (VI) from aqueous solutions by manganese oxide coated zeolite: discussion of adsorption isotherms and pH effect. J. Environ. Radioact. 93(3), 127–143 (2007). https://doi.org/10.1016/j.jenvrad.2006.12.003
S. Gabriel, A. Baschwitz, G. Mathonniere et al., A critical assessment of global uranium resources, including uranium in phosphate rocks, and the possible impact of uranium shortages on nuclear power fleets. Ann. Nucl. Energy 58, 13–220 (2013). https://doi.org/10.1016/j.anucene.2013.03.010
M.A. Mousa, H.S. Gado, M.M.G. Abd-El Fattah et al., Removal of uranium from crude phosphoric acid by precipitation technique. Arab J. Nucl. Sci. Appl. 46, 38–47 (2013)
A. Leydier, G. Arrachart, T. Raphael et al., Recovery of uranium (VI) from concentrated phosphoric acid using bifunctional reagents. Hydrometallurgy 171, 262–266 (2017). https://doi.org/10.1016/j.hydromet.2017.05.008
N.T. El-Hazek, M.S. El-Sayed, Direct uranium extraction from dihydrate and hemi-dihydrate wet process phosphoric acids by liquid emulsion membrane. J. Radioanal. Nucl. Chem. 257, 347–352 (2003)
M.M. Aly, M.A. Mousa, M.H. Taha et al., Kinetics and thermodynamics of uranium adsorption from commercial di-hydrate phosphoric acid using D2EHPA-impregnated charcoal. Arab J. Nucl. Sci. Appl. 46, 29–37 (2013)
H.F. Ali, M.M. Ali, M.H. Taha et al., Uranium extraction mechanism from analytical grade phosphoric acid using D2EHPA and synergistic D2EHPA-TOPO mixture. Int. J. Nucl. Energy Sci. Eng. 2, 57–61 (2012)
M. Saeed, M. Munir, M. Nafees et al., Synthesis, characterization and applications of silylation based grafted bentonite for the removal of Sudan dyes: isothermal, kinetic and thermodynamic studies. Microporous Mesoporous Mater. 291, 109697 (2020). https://doi.org/10.1016/j.micromeso.2019.109697
A.M. Masoud, M. Saeed, M.H. Taha et al., Uranium adsorption from Bahariya Oasis leach liquor via TOPO impregnated bentonite material; Isothermal, kinetic and thermodynamic studies. Egypt. J. Chem. (2019). https://doi.org/10.21608/EJCHEM.2019.13638.1843
A.M.A. El Naggar, M.M. Ali, S.A. Abdel Maksoud et al., Waste generated bio-char supported co-nanoparticles of nickel and cobalt oxides for efficient adsorption of uranium and organic pollutants from industrial phosphoric acid. J. Radioanal. Nucl. Chem. 320(3), 741–755 (2019)
J.P. McKinley, J.M. Zachara, S.C. Smith et al., The influence of uranyl hydrolysis and multiple site-binding reactions on adsorption of U (VI) to montmorillonite. Clays Clay Miner. 43(5), 586–598 (1995). https://doi.org/10.1346/CCMN.1995.0430508
M.H. Taha, M.M. El-Maadawy, A.E.M. Hussein et al., Uranium sorption from commercial phosphoric acid using kaolinite and metakaolinite. J. Radioanal. Nucl. Chem. 317(2), 685–699 (2018)
S. Aytas, S. Akyil, M. Eral, Adsorption and thermodynamic behavior of uranium on natural zeolite. J. Radioanal. Nucl. Ch. 260(1), 119–125 (2004). https://doi.org/10.1023/B:JRNC.0000027070.25215.92
M. Bonato, K.V. Ragnarsdottir, G.C. Allen, Removal of uranium (VI), lead (II) at the surface of TiO2 nanotubes studied by X-ray photoelectron spectroscopy. Water Air Soil Pollut. 223(7), 3845–3857 (2012). https://doi.org/10.1007/s11270-012-1153-1
Z. Guo, Z. Yan, Z. Tao, Sorption of uranyl ions on TiO2: effects of contact time, ionic strength, concentration and humic substance. J. Radioanal. Nucl. Ch. 261(1), 157–162 (2004). https://doi.org/10.1023/B:JRNC.0000030950.09543.2e
H. Zhang, Y. Xie, Z. Tao, Sorption of uranyl ions on gibbsite: effects of contact time, pH, ionic strength, concentration and anion of electrolyte. Colloids Surf. A 252(1), 1–5 (2005). https://doi.org/10.1016/j.colsurfa.2004.10.005
G.L. Drisko, M.C. Kimling, N. Scales et al., One-pot preparation and uranyl adsorption properties of hierarchically porous zirconium titanium oxide beads using phase separation processes to vary macropore morphology. Langmuir 26(22), 17581–17588 (2010). https://doi.org/10.1021/la103177h
X. Guo, R. Chen, Q. Liu et al., Graphene oxide and silver ions coassisted zeolitic imidazolate framework for antifouling and uranium enrichment from seawater. ACS Sustain. Chem. Eng. 7(6), 6185–6195 (2019). https://doi.org/10.1021/acssuschemeng.8b06391
J. Zhu, Q. Liu, J. Liu et al., Ni–Mn LDH-decorated 3D Fe-inserted and N-doped carbon framework composites for efficient uranium (VI) removal. Environ. Sci. Nano 5, 467–475 (2018). https://doi.org/10.1039/C7EN01018D
L. Tan, Y. Wang, Q. Liu et al., Enhanced adsorption of uranium (VI) using a three-dimensional layered double hydroxide/graphene hybrid material. Chem. Eng. J. 259, 752–760 (2015). https://doi.org/10.1016/j.cej.2014.08.015
V. Stucker, J.F. Ranville, M. Newman et al., Evaluation and application of anion exchange resins to measure groundwater uranium flux at a former uranium mill site. Water Res. 45(16), 4866–4876 (2011). https://doi.org/10.1016/j.watres.2011.06.030
E. Rosenberg, G. Pinson, R. Tsosie et al., Uranium remediation by ion exchange and sorption methods: a critical review. Johnson Matthey Tech. 60, 59–77 (2016). https://doi.org/10.1595/205651316X690178
B. Gu, Y.-K. Ku, G.M. Brown, Sorption and desorption of perchlorate and U (VI) by strong-base anion-exchange resins. Environ. Sci. Technol. 39(3), 901–907 (2005). https://doi.org/10.1021/es049121f
R. Hoffmann, S. Alvarez, C. Mealli et al., From widely accepted concepts in coordination chemistry to inverted ligand fields. Chem. Rev. 116(14), 8173–8192 (2016). https://doi.org/10.1021/acs.chemrev.6b00251
A. Umar, M. Munir, M. Murtaza et al., Properties and green applications based review on highly efficient deep eutectic solvents. Egypt. J. Chem. (2019). https://doi.org/10.21608/ejchem.2019.12604.1782
R. Buonsanti, D.J. Milliron, Chemistry of doped colloidal nanocrystals. Chem. Mater. 25(8), 1305–1317 (2013). https://doi.org/10.1021/cm304104m
C. Goupil, W. Seifert, K. Zabrocki et al., Thermodynamics of thermoelectric phenomena and applications. Entropy 13(8), 1481–1517 (2011). https://doi.org/10.3390/e13081481
M. Soleilhavoup, G. Bertrand, Cyclic (alkyl)(amino) carbenes (CAACs): stable carbenes on the rise. Acc. Chem. Res. 48(2), 256–266 (2014). https://doi.org/10.1021/ar5003494
M.C. Kim, G.S. Hwang, R.S. Ruoff, Epoxide reduction with hydrazine on graphene: a first principles study. J. Chem. Phys. 131(6), 064704 (2009). https://doi.org/10.1063/1.3197007
G. Chattopadhyay, P.S. Ray, Hydrazine-hydroquinone complex as an efficient solid phase hydrazine donor: high yield synthesis of luminol and isoluminol. J. Chem. Res. 35(6), 326–328 (2011). https://doi.org/10.3184/174751911X13058077114827
J.A. Dean, Lange’s Handbook of Chemistry. (McGraw-Hill Inc, New york, 1999)
S. Sangeetha, V. Ranjithkumar, S. Rajendran et al., Synthesis, structure and thermal behavior of hydrazine-coordinated copper pyromellitate polymeric complex and dihydrazinium pyromellitate. J. Therm. Anal. Calorim. 124, 1601–1608 (2016). https://doi.org/10.1007/s10973-015-5206-8
M. Gustafsson, A. Fischer, A. Ilyukhin et al., Novel polynuclear nickel (II) complex: hydrazine, sulfato, and hydroxo bridging in an unusual metal hexamer crystal structure and magnetic properties of [Ni6(N2H4)6(SO4)4(OH)2(H2O)8](SO4)(H2O)10. Inorg. Chem. 49(12), 5359–5361 (2010). https://doi.org/10.1021/ic100648c
E.H.P. Bai, S. Vairam, Spectral and thermal studies of transition metal complexes of Acetamido Benzoic Acids with hydrazine. Asian J. Chem. 25(1), 209 (2013)
H.K. James, Y. Miron, H.E. Perlee, Physical and Explosion Characteristics of Hydrazine Nitrate (Bureau of Mines, Washington, DC, 1970)
A.L. Johnson, N. Hollingsworth, A. Kingsley et al., New organocadmium hydrazine adducts and hydrazide complexes. Eur. J. Inorg. Chem. 2012(2), 246–250 (2012). https://doi.org/10.1002/ejic.201100885
B. Raju, B.N. Sivasankar, Spectral, thermal and X-ray studies on some new Bis-hydrazine lanthanide(III) glyoxylates. J. Therm. Anal. Calorim. 94, 289–296 (2008). https://doi.org/10.1007/s10973-007-8953-3
M. Talawar, A.P. Agrawal, J.S. Chhabra et al., Studies on nickel hydrazinium nitrate (NHN) and bis-(5-nitro-2H tetrazolato-N2) tetraamino cobalt (III) perchlorate (BNCP): potential lead-free advanced primary explosives. J. Sci. Ind. Res. 63(8), 677–681 (2004)
L. Vikram, B.N. Sivasankar, New hydrazinium complexes of lanthanide (III) with ethylenediaminetetraacetate: spectral, thermal and XRD studies. Indian J. Chem. Sect. A 45(4), 864–871 (2006)
N.M. Farag, G.O. El-sayed, A.M.A. Morsy et al., Modification of Davies Gray method for uranium determination in phosphoric acid solutions. Int. J. Adv. Res. 3, 323–337 (2015)
R.N. Mendoza, T.I. Saucedo Medina, A. Vera et al., Study of the sorption of Cr(III) with XAD-2 resin impregnated with di-(2, 4, 4 trimethylpentyl) phosphinicacid (Cyanex 272). Solv. Extr. Ion Exch. 18(2), 319–343 (2000). https://doi.org/10.1080/07366290008934684
H. Ullah, M. Nafees, F. Iqbal et al., Adsorption kinetics of malachite green and methylene blue from aqueous solutions using surfactant-modified organoclays. Acta Chim. Slov. 64(2), 449–460 (2017). https://doi.org/10.17344/acsi.2017.3285
M. Munir, M. Ahmad, M. Saeed et al., Sustainable production of bioenergy from novel non-edible seed oil (Prunus cerasoides) using bimetallic impregnated montmorillonite clay catalyst. Renew. Sustain. Energy Rev. 109, 321–332 (2019). https://doi.org/10.1016/j.rser.2019.04.029
M.T. Akhtar, M. Ahmad, A. Shaheen et al., Comparative study of liquid biodiesel from sterculia foetida (Bottle Tree) using CuO-CeO2 and Fe2O3 nano catalysts. Frontiers in Energy Research 7(4), 1–15 (2019). https://doi.org/10.3389/fenrg.2019.00004
A.E.M. Hussein, A.M.A. Morsy, Uranium recovery from wet-process phosphoric acid by a commercial ceramic product. Arab. J. Chem. 10, S361–S367 (2017). https://doi.org/10.1016/j.arabjc.2012.09.007
Z.-J. Yi, J. Yao, Y.-F. Kuang et al., Uptake of hexavalent uranium from aqueous solutions using coconut husk activated carbon. Desalinat. Water Treat. 57(4), 1749–1755 (2015). https://doi.org/10.1080/19443994.2014.977956
M.T. Uddin, M.S. Islam, M.Z. Abedin, Adsorption of phenol from aqueous solution by water hyacinth ash. ARPN J. Eng. Appl. Sci. 2(2), 11–17 (2007)
C. Kütahyalı, M. Eral, Selective adsorption of uranium from aqueous solutions using activated carbon prepared from charcoal by chemical activation. Sep. Purif. Technol. 40(2), 109–114 (2004). https://doi.org/10.1016/j.seppur.2004.01.011
A. Kausar, H.N. Bhatti, G. MacKinnon, Equilibrium, kinetic and thermodynamic studies on the removal of U (VI) by low cost agricultural waste. Colloids Surf. B 2013(111), 124–133 (2013). https://doi.org/10.1016/j.colsurfb.2013.05.028
M. Solgy, M. Taghizadeh, D. Ghoddocynejad, Adsorption of uranium (VI) from sulphate solutions using Amberlite IRA-402 resin: equilibrium, kinetics and thermodynamics study. Ann. Nucl. Energy 75, 132–138 (2015). https://doi.org/10.1016/j.anucene.2014.08.009
S. Yusan, S.A. Erenturk, Adsorption equilibrium and kinetics of U (VI) on beta type of akaganeite. Desalination 263(1–3), 233–239 (2010). https://doi.org/10.1016/j.desal.2010.06.064
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morsy, A., Taha, M.H., Saeed, M. et al. Isothermal, kinetic, and thermodynamic studies for solid-phase extraction of uranium (VI) via hydrazine-impregnated carbon-based material as efficient adsorbent. NUCL SCI TECH 30, 167 (2019). https://doi.org/10.1007/s41365-019-0686-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41365-019-0686-z