Abstract
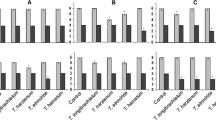
Colletotrichum nymphaeae is the principal causal agent of strawberry anthracnose worldwide, including Iran. For disease management, eco-friendly alternatives such as biological control instead of chemical fungicides are highly desirable. The aim of this study was to compare the efficacy of 20 strains of Trichoderma spp. indigenous to Iran against C. nymphaeae, to a commercial strain of Trichoderma atroviride (SC1) isolated elsewhere, under in vitro and in planta conditions. The tested strains belonged to T. harzianum (ten isolates), T. pleuroticola (five isolates), T. virens (three isolates), T. asperellum (one isolate) and T. afroharzianum (one isolate). In vitro results showed that most isolates used in this study were able to significantly inhibit mycelial growth of the pathogen in dual culture and non-volatile tests. The majority of the tested Trichoderma spp. isolates produced lytic enzymes, including chitinase, β-1,3-glucanase, protease and cellulase. Besides producing hydrogen cyanide, Trichoderma spp. strains were also shown to be positive in terms of siderophore production and the mycoparasitism process. In planta bioassays revealed that the best indigenous strain (T. virens CCTUT4) significantly reduced anthracnose, at the same rate as the commercial strain, although the standard chemical fungicide (Switch®) was superior in terms of disease control. A positive correlation between disease incidence and severity reduction was detected with increasing population density of the antagonists in this study. The findings of this study indicate that isolating Trichoderma-based biocontrol agents from the crop against whose disease are targeted does not necessarily provide advantages in comparison with a strain having good biocontrol properties and originating from a different substrate.






Similar content being viewed by others
References
Food and Agriculture Organization (FAO). (2007). http://faostat3.fao.org/download/Q/QC/E.
Wang, S. Y., & Lewers, K. S. (2007). Antioxidant capacity and flavonoid content in wild strawberries. Journal of the American Society for Horticultural Science, 132(5), 629–637.
Mass, J. L. (1998). Compendium of strawberry diseases. St. Paul, MN: APS Press.
Freeman, S., & Katan, T. (1997). Identification of Colletotrichum species responsible for anthracnose and root necrosis of strawberry in Israel. Phytopathology, 87, 516–521.
Howard, C. M., Maas, J. L., Chandler, C. K., & Albregts, E. E. (1992). Anthracnose of strawberry caused by the Colletotrichum complex in Florida. Plant Disease, 76, 976–981.
Damm, U., Cannon, P. F., Woudenberg, J. H. C., & Crous, P. W. (2012). The Colletotrichum acutatum species complex. Studies in Mycology, 73, 37–114.
Han, Y. C., Zeng, X. G., Xiang, F. Y., Ren, L., Chen, F. Y., & Gu, Y. C. (2016). Distribution and characteristics of Colletotrichum spp. associated with anthracnose of strawberry in Hubei, China. Plant Disease, 100(5), 996–1006.
Weir, B. S., Johnston, P. R., & Damm, U. (2012). The Colletotrichum gloeosporioides species complex. Studies in Mycology, 73, 115–180.
Baroncelli, R., Zapparata, A., Sarrocco, S., Sukno, S. A., Lane, C. R., Thon, M. R., et al. (2015). Molecular diversity of anthracnose pathogen populations associated with UK strawberry production suggests multiple introductions of three different Colletotrichum species. PLoS ONE, 10(6), 0129140.
Karimi, K., Babai Ahari, A., Arzanlou, M., Amini, J., Pertot, I., & Rota-Stabelli, O. (2016). Application of the consolidated species concept to identify the causal agent of strawberry anthracnose in Iran and initial molecular dating of the Colletotrichum acutatum species complex. European Journal of Plant Pathology, 147, 375–387.
Živković, S. V., Stojanović, S., Ivanović, Ž., Gavrilović, V., Popović, T. A., & Balaž, J. E. (2010). Screening of antagonistic activity of microorganisms against Colletotrichum acutatum and Colletotrichum gloeosporioides. Archives of Biological Science Belgrade, 62, 611–623.
Elad, Y., Barbul, O., Nitzani, Y., Rav David, D., Zveibil, A., Maimon, M., et al. (2001). Inter- and intra-species variation in bio-control activity. In: Proceedings of the 5th congress of the European foundation of plant pathology (pp. 474–478).
Freeman, S., Barbul, O., Rav David, D., Nitzani, Y., Zveibil, A., & Elad, Y. (2001). Trichoderma spp. for biocontrol of Colletotrichum acutatum and Botrytis cinerea in strawberry. Biocontrol of Fungal and Bacterial Plant Pathogens, IOBC/WPRS Bulletin, 24, 147–150.
Freeman, S., Minz, D., Kolesnik, I., Barbul, O., Zveibil, A., Maymon, M., et al. (2004). Trichoderma biocontrol of Colletotrichum acutatum and Botrytis cinerea and survival in strawberry. European Journal of Plant Pathology, 110, 361–370.
Verma, N., MacDonald, L., & Punja, Z. K. (2006). Inoculum prevalence, host infection and biological control of Colletotrichum acutatum: Causal agent of blueberry anthracnose in British Columbia. Plant Pathology, 55, 442–450.
Porras, M., Barrau, C., & Romero, F. (2009). Influence of Trichoderma and soil solarization on strawberry yield. Acta Horticulture, 842, 389–392.
Chaverri, P., Branco-Rocha, F., Jaklitsch, W., Gazis, R., Degenkolb, T., & Samuels, G. J. (2015). Systematics of the Trichoderma harzianum species complex and the re-identification of commercial biocontrol strains. Mycologia, 3, 558–590.
Pertot, I., Gobbin, D., De Luca, F., & Prodorutti, D. (2008). Methods of assessing the incidence of Armillaria root rot across viticultural areas and the pathogen’s genetic diversity and spatial–temporal pattern in northern Italy. Crop Protection, 27, 1061–1070.
Pertot, I., Prodorutti, D., Colombini, A., & Pasini, L. (2016). Trichoderma atroviride SC1 prevents Phaeomoniella chlamydospora and Phaeoacremonium aleophilum infection of grapevine plants during the grafting process in nurseries. BioControl, 61, 257–267.
Handelsman, J., & Stabb, E. V. (1996). Biocontrol of soilborn plant pathogens. The Plant Cell, 8, 1855–1869.
Narisawa, K., Ohki, K. T., & Hashiba, T. (2000). Suppression of clubroot and Verticillium yellows in Chinese cabbage in the field by the root endophytic fungus, Heteroconium chaetospira. Plant Pathology, 49, 141–146.
Karimi, K., Babai Ahari, A., Weisany, W., Pertot, I., Vrhovsek, U., & Arzanlou, M. (2016). Funneliformis mosseae root colonization affects Anethum graveolens essential oil composition and its efficacy against Colletotrichum nymphaeae. Industrial Crops and Products, 90, 126–134.
Arzanlou, M., Bakhshi, M., Karimi, K., & Torbati, M. (2015). Multigene phylogeny reveals three new records of Colletotrichum spp. and several new host records for the mycobiota of Iran. Journal of Plant Protection Research, 55(2), 198–211.
White, T. J., Bruns, T., & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, & J. W. White (Eds.), A guide to molecular methods and applications (p. 482). New York: Academic Press.
Staden, R. (1996). The staden sequence analysis package. Molecular Biotechnology, 5(3), 233–241.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., & Kumar, S. (2013). MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30, 2725–2729.
Nylander, J. A. A. (2004). MrModeltest v2. Program distributed by the author. Uppsala: Evolutionary Biology Centre, Uppsala University.
Ronquist, F., & Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19, 1572–1574.
Jaklitsch, W. M., & Voglmayr, H. (2015). Biodiversity of Trichoderma (Hypocreaceae) in Southern Europe and Macaronesia. Studies in Mycology, 80, 1–87.
Dennis, C., & Webster, J. (1971). Antagonistic properties of species groups of Trichoderma II. Production of volatile antibiotics. Transactions of British Mycological Society, 57, 41–48.
Dennis, C., & Webster, J. (1971). Antagonistic properties of species groups of Trichoderma I. Production of non-volatile antibiotics. Transactions of British Mycological Society, 57, 25–39.
Karimi, K., Amini, J., Harighi, B., & Bahramnejad, B. (2012). Evaluation of biocontrol potential of Pseudomonas and Bacillus spp. against Fusarium wilt of chickpea. Australian Journal of Crop Science, 6(4), 695–703.
Kasana, R. C., Salwan, R., Dhar, H., Dutt, S., & Gulati, A. (2008). Arapid and easy method for the detection of microbial cellulases on agar plates using gram’s iodine. Current Microbiology, 57, 503–507.
Monreal, J., & Reese, E. T. (1969). The chitinase of Serratia marcescens. Canadian Journal of Microbiology, 15, 669–689.
Agrawal, T., & Kotasthane, A. S. (2012). Chitinolytic assay of indigenous Trichoderma isolates collected from different geographical locations of Chhattisgarh in Central India. SpringerPlus, 1(1), 1.
Alstrom, S. (1987). Factors associated with detrimental effects of rhizobacteria on plant growth. Plant and Soil, 102, 3–9.
Schwyn, B., & Neilands, J. B. (1997). Universal chemical assay for the detection and determination of siderophores. Analytical Biochemistry, 160, 46–56.
Mertely, J. C., & Legard, D. E. (2004). Detection, isolation, and pathogenecity of Colletotrichum spp. from strawberry petioles. Plant Disease, 88, 407–412.
Davet, P., & Rouxel, F. (2000). Detection and isolation of soil fungi. In K. T. Kaimoni (Ed.), Soil fungi-laboratory manuals (p. 188). USA: Science Publishers.
Weller, D. M. (1988). Biological control of soil born plant pathogens in the rhizosphere with bacteria. Annual Review of Phytopathology, 26, 379–407.
Benítez, T., Rincón, A. M., Limón, M. C., & Codón, A. C. (2004). Biocontrol mechanisms of Trichoderma strains. International Microbiology, 7, 249–260.
Druzhinina, I. S., Kopchinskiy, A. G., & Kubicek, C. P. (2006). The first 100 Trichoderma species characterized by molecular data. Mycoscience, 47(2), 55–64.
Jiang, Y., Wang, J. L., Chen, J., Mao, L. J., Feng, X.-X., Zhang, C. L., et al. (2016). Trichoderma biodiversity of agricultural fields in east china reveals a gradient distribution of species. PLoS ONE, 11(8), e0160613.
Maina, P. K., Wachira, P. M., Okoth, S. A., & Kimenju, J. W. (2015). Distribution and diversity of indigenous Trichoderma species in Machakos county, Kenya. British Microbiology Research Journal, 9(4), 1–15.
Sariah, M., Choo, C. W., Zakaria, H., & Norihan, M. S. (2005). Quantification and characterisation of Trichoderma spp. from different ecosystems. Mycopathology, 159(1), 113–117.
Stutz, E. W., Défago, G., & Kern, H. (1986). Naturally occurring fluorescent pseudomonads involved in suppression of black root rot of tobacco. Phytopathology, 76(2), 181–185.
Keel, C., Weller, D. M., Natsch, A., Défago, G., Cook, R. J., & Thomashow, L. S. (1996). Conservation of the 2, 4-diacetylphloroglucinol biosynthesis locus among fluorescent Pseudomonas strains from diverse geographic locations. Applied and Environmental Microbiology, 62(2), 552–563.
Kamala, Th, & Indira, S. (2011). Evaluation of indigenous Trichoderma isolates from Manipur as biocontrol agent against Pythium aphanidermatum on common beans. 3 Biotech, 1, 217–225.
Viterbo, A., Haran, S., Friesem, D., Ramot, O., & Chet, I. (2001). Antifungal activity of a novel endochitinase gene (chit36) from Trichoderma harzianum Rifai TM. FEMS Microbiology Letters, 200, 169–174.
Haran, S., Schikler, H., & Chet, I. (1996). Molecular mechanisms of lytic enzymes involved in the biocontrol activity of Trichoderma harzianum. Microbiology, 142, 2321–2331.
de los Santos-Villalobos, S., Guzmán-Ortiz, D. A., Gómez-Lim, M. A., Délano-Frier, J. P., de-Folter, S., Sánchez-García, P., et al. (2013). Potential use of Trichoderma asperellum (Samuels, Liechfeldt et Nirenberg) T8a as a biological control agent against anthracnose in mango (Mangifera indica L.). Biological Control, 64(1), 37–44.
Begum, M. M., Sariah, M., Zainal Abidin, M. A., Puteh, A. B., & Rahman, M. A. (2008). antagonistic potential of selected fungal and bacterial biocontrol agents against Colletotrichum truncatum of soybean seeds. Pertanika Journal of Tropical Agricultural Science, 31(1), 45–53.
Gravel, V., Antoun, H., & Tweddell, R. J. (2007). Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: Possible role of indole acetic acid (IAA). Soil Biology and Biochemistry, 39, 1968–1977.
Leong, J. (1986). Siderophores: Their biochemistry and possible role in the biocontrol of plant pathogens. Annual review of Phytopathology, 24, 187–209.
Dwivedi, D., & Johri, B. N. (2003). Antifungals from fluorescent pseudomonads: Biosynthesis and regulation. Current Science, 12, 1693–1703.
Elad, Y., & Baker, R. (1985). The role of competition for iron and carbon in suppression of chlamydospore germination of Fusarium spp. by Pseudomonas spp. Phytopathology, 75, 1053–1059.
Kloepper, J. W., Leong, J., Teintze, M., & Schroth, M. N. (1980). Pseudomonas siderophores: A mechanism explaining disease-suppressive soils. Current Microbiology, 4, 317–320.
Tortora, M. L., Díaz-Ricci, J. C., & Pedraza, R. O. (2011). Azospirillum brasilense siderophores with antifungal activity against Colletotrichum acutatum. Archives of Microbiology, 193(4), 275–286.
Anand, T., Chandrasekaran, A., Kuttalam, S., Senthilraja, G., & Samiyappan, R. (2010). Integrated control of fruit rot and powdery mildew of chilli using the biocontrol agent Pseudomonas fluorescens and a chemical fungicide. Biological Control, 52(1), 1–7.
Deberdt, P., Mfegue, C. V., Tondje, P. R., Bon, M. C., Ducamp, M., Hurard, C., et al. (2008). Impact of environmental factors, chemical fungicide and biological control on cacao pod production dynamics and black pod disease (Phytophthora megakarya) in Cameroon. Biological Control, 44(2), 149–159.
Gachango, E., Kirk, W., Schafer, R., & Wharton, P. (2012). Evaluation and comparison of biocontrol and conventional fungicides for control of postharvest potato tuber diseases. Biological Control, 63(2), 115–120.
Ilhan, K., & Karabulut, O. A. (2013). Efficacy and population monitoring of bacterial antagonists for gray mold (Botrytis cinerea Pers. ex. Fr.) infecting strawberries. Biological Control, 58, 457–470.
Ponmurugan, P., & Baby, U. I. (2007). Evaluation of fungicides and biocontrol agents against Phomopsis canker of tea under field conditions. Australasian Plant Pathology, 36(1), 68–72.
Peres, N. A., Seijo, T. E., & Turechek, W. W. (2010). Pre-and post-inoculation activity of a protectant and a systemic fungicide for control of anthracnose fruit rot of strawberry under different wetness durations. Crop Protection, 29(10), 1105–1110.
Wedge, D. E., Smith, B. J., Quebedeaux, J. P., & Constantin, R. J. (2007). Fungicide management strategies for control of strawberry fruit rot diseases in Louisiana and Mississippi. Crop Protection, 26(9), 1449–1458.
Savazzini, F., Longa, C. M. O., Pertot, I., & Gessler, C. (2008). Real-time PCR for detection and quantification of the biocontrol agent Trichoderma atroviride strain SC1 in soil. Journal of Microbiological Methods, 73, 185–194.
Longa, C. M. O., Savazzini, F., Tosi, S., Elad, Y., & Pertot, I. (2009). Evaluating the survival and environmental fate of the biocontrol agent Trichoderma atroviride SC1 in vineyards in northern Italy. Journal of applied microbiology, 106(5), 1549–1557.
Acknowledgements
The authors would like to thank the Research Deputy of the University of Tabriz and the EU INNOVA project (FP7-People-2012-IAPP, Grant Agreement 324416).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Karimi, K., Babai Ahari, A., Arzanlou, M. et al. Comparison of indigenous Trichoderma spp. strains to a foreign commercial strain in terms of biocontrol efficacy against Colletotrichum nymphaeae and related biological features. J Plant Dis Prot 124, 453–466 (2017). https://doi.org/10.1007/s41348-017-0088-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-017-0088-6