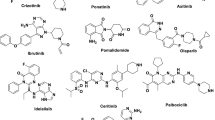
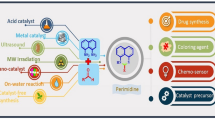
Abstract
The Diels–Alder reaction (DAR) is found in myriad applications in organic synthesis and medicinal chemistry for drug development, as it is the method of choice for the expedient synthesis of complex natural compounds and innovative materials including nanomaterials, graphene expanses, and polymeric nanofibers. Furthermore, the greatest focus of attention of DARs is on the consistent reaction procedure with stimulus yields by highly stereo- and regioselective mechanistic pathways. Therefore, the present review is intended to summarize conventional solvent-free (SF) DARs for the expedient synthesis of heterocyclic compounds and materials. In particular, this review deals with the DARs of mechanochemical grinding, catalysis (including stereoselective catalysts), thermal, and electromagnetic radiation (such as microwave [MW], infrared [IR], and ultraviolet [UV] irradiation) in SF procedures. Therefore, this comprehensive review validates the application of DARs to pharmaceutical innovations and biorenewable materials through consistent synthetic approaches.
Graphical Abstract






































Similar content being viewed by others
References
Cao MH, Green NJ, Xu SZ (2017) Application of the aza-Diels–Alder reaction in the synthesis of natural products. Org Biomol Chem 15(15):3105–3129. https://doi.org/10.1039/C6OB02761J
Houk KN, Liu F, Yang Z, Seeman JI (2021) Evolution of the Diels–Alder reaction mechanism since the 1930s: Woodward, Houk with Woodward, and the influence of computational chemistry on understanding cycloadditions. Angew Chem Int Ed 60(23):12660–12681. https://doi.org/10.1002/anie.202001654
Huang G, Kouklovsky C, de la Torre A (2020) Inverse-electron-demand Diels–Alder reactions of 2-pyrones: bridged lactones and beyond. Chem Eur J 27:4760–4788. https://doi.org/10.1002/chem.202003980
Pałasz A (2016) Recent advances in inverse-electron-demand hetero-Diels–Alder reactions of 1-oxa-1, 3-butadienes. Top Curr Chem 374(3):1–37. https://doi.org/10.1007/s41061-016-0026-2
Carey FA, Sundberg RJ (2007) Advanced organic chemistry: part B: reaction and synthesis, 5th edn. Springer, New York. https://doi.org/10.1007/978-0-387-44899-2
Eschenbrenner-Lux V, Kumar K, Waldmann H (2014) The asymmetric hetero-Diels–Alder reaction in the syntheses of biologically relevant compounds. Angew Chem Int Ed 53(42):11146–11157. https://doi.org/10.1002/anie.201404094
Kotha S, Chavan AS, Goyal D (2019) Diversity-oriented approaches to polycycles and heterocycles via enyne metathesis and Diels–Alder reaction as key steps. ACS Omega 4(27):22261–22273. https://doi.org/10.1021/acsomega.9b03020
Li W, Zhou L, Zhang J (2016) Recent progress in dehydro (genative) Diels–Alder reaction. Chem Euro J 22(5):1558–1571. https://doi.org/10.1002/chem.201503571
Windmon N, Dragojlovic V (2008) Diels–Alder reactions in the presence of a minimal amount of water. Green Chem Lett Rev 1(3):155–163. https://doi.org/10.1080/17518250802482505
Gregoritza M, Brandl FP (2015) The Diels–Alder reaction: a powerful tool for the design of drug delivery systems and biomaterials. Eur J Pharm Biopharm 97:438–453. https://doi.org/10.1016/j.ejpb.2015.06.007
Kurpanik A, Matussek M, Lodowski P, Szafraniec-Gorol G, Krompiec M, Krompiec S (2020) Diels–Alder cycloaddition to the bay region of perylene and its derivatives as an attractive strategy for PAH core expansion: theoretical and practical aspects. Molecules 25(22):5373. https://doi.org/10.3390/molecules25225373
Oluwasanmi A, Hoskins C (2021) Potential use of the Diels–Alder reaction in biomedical and nanomedicine applications. Int J Pharm. https://doi.org/10.1016/j.ijpharm.2021.120727
Negri V, Pacheco-Torres J, Calle D, López-Larrubia P (2020) Carbon nanotubes in biomedicine. Top Curr Chem (Z) 378:15. https://doi.org/10.1007/s41061-019-0278-8
Stolle A, Szuppa T, Leonhardt SE, Ondruschka B (2011) Ball milling in organic synthesis: solutions and challenges. Chem Soc Rev 40(5):2317–2329. https://doi.org/10.1039/C0CS00195C
Wang GW (2013) Mechanochemical organic synthesis. Chem Soc Rev 42(18):7668. https://doi.org/10.1039/C3CS35526H
Gonnet L, Chamayou A, André-Barrès C, Micheau JC, Guidetti B, Sato T, Baron M, Baltas M, Calvet R (2021) Elucidation of the Diels–Alder reaction kinetics between diphenylfulvene and maleimide by mechanochemistry and in solution. ACS Sustain Chem Eng 9(12):4453–4462. https://doi.org/10.1021/acssuschemeng.0c08314
Clarke PA, Santos S, Martin WH (2007) Combining pot, atom and step economy (PASE) in organic synthesis. Synthesis of tetrahydropyran-4-ones. Green Chem 9(5):438–440. https://doi.org/10.1039/B700923B
Margetic D, Štrukil V (2016) Mechanochemical organic synthesis. Elsevier. https://doi.org/10.1016/C2014-0-01621-8
Lavanya M, Lin C, Mao J, Thirumalai D, Aabaka SR, Yang X, Mao J, Huang Z, Zhao J (2021) Synthesis and anticancer properties of functionalized 1, 6-naphthyridines. Top Curr Chem 379(2):1–75. https://doi.org/10.1007/s41061-020-00314-6
Rammohan A, Reddy JS, Sravya G, Rao CN, Zyryanov GV (2020) Chalcone synthesis, properties and medicinal applications: a review. Environ Chem Lett 18:433–458. https://doi.org/10.1007/s10311-019-00959-w
Achar TK, Bose A, Mal P (2017) Mechanochemical synthesis of small organic molecules. Beilstein J Org Chem 13(1):1907–1931. https://doi.org/10.3762/bjoc.13.186
Stevenson R, De Bo G (2017) Controlling reactivity by geometry in retro-Diels–Alder reactions under tension. J Am Chem Soc 139(46):16768–16771. https://doi.org/10.1021/jacs.7b08895
Zhang Z, Peng ZW, Hao MF, Gao JG (2010) Mechanochemical Diels–Alder cycloaddition reactions for straightforward synthesis of endo-norbornene derivatives. Synlett 19:2895–2898. https://doi.org/10.1055/s-0030-1259030
Do JL, Friščić T (2017) Mechanochemistry: a force of synthesis. ACS Cent Sci 3(1):13. https://doi.org/10.1021/acscentsci.6b00277
McKissic KS, Caruso JT, Blair RG, Mack J (2014) Comparison of shaking versus baking: further understanding the energetics of a mechanochemical reaction. Green Chem 16(3):1628–1632. https://doi.org/10.1039/C3GC41496E
Suryanarayana C (2001) Mechanical alloying and milling. Progr Mater Sci 46(1–2):1–184. https://doi.org/10.1016/S0079-6425(99)00010-9
Maleki A, Javanshir S, Naimabadi M (2014) Facile synthesis of imidazo [1, 2-a] pyridines via a one-pot three-component reaction under solvent-free mechanochemical ball-milling conditions. RSC Adv 4(57):30229–30232. https://doi.org/10.1039/C3RA43221A
Agarwal J, Rani R, Peddinti RK (2017) Mechanochemical grinding Diels–Alder reaction: highly efficient and rapid access to bi-, tri-, and tetracyclic systems. Synlett 28(11):1336–1340. https://doi.org/10.1055/s-0036-1558970
Wang FJ, Xu H, Xin M, Zhang Z (2016) I2-mediated amination/cyclization of ketones with 2-aminopyridines under high-speed ball milling: solvent-and metal-free synthesis of 2,3-substituted imidazo[1,2-a]pyridines and zolimidine. Mol Divers 20(3):659–666. https://doi.org/10.1007/s11030-016-9666-y
Suri M, Hussain FL, Gogoi C, Das P, Pahari P (2020) Magnetically recoverable silica catalysed solvent-free domino Knoevenagel-hetero-Diels–Alder reaction to access divergent chromenones. Org Biomol Chem 18(11):2058–2062. https://doi.org/10.1039/D0OB00284D
Ando RA, Junior GAB, Brocksom TJ, Donatoni MC, de Oliveira KT, Dos Santos AA (2014) Solvent-free Diels–Alder reactions catalyzed by FeCl3 on Aerosil ((R)) silica. Tetrahedron 70(20):3231–3238. https://doi.org/10.1016/j.tet.2014.02.017
Soleimani Amiri S (2020) Green production and antioxidant activity study of new pyrrolo [2, 1-a] isoquinolines. J Heterocyclic Chem 57(11):4057–4069. https://doi.org/10.1002/jhet.4115
Jiang Y, Chen CF (2011) Recent developments in synthesis and applications of triptycene and pentiptycene derivatives. Eur J Org Chem 2011(32):6377–6403. https://doi.org/10.1002/ejoc.201100684
Zyryanov GV, Palacios MA, Anzenbacher P Jr (2008) Simple molecule-based fluorescent sensors for vapor detection of TNT. Org Lett 10(17):3681–3684. https://doi.org/10.1021/ol801030u
Zhao Y, Rocha SV, Swager TM (2016) Mechanochemical synthesis of extended iptycenes. J Am Chem Soc 138(42):13834–13837. https://doi.org/10.1021/jacs.6b09011
Tan YJ, Zhang Z, Wang FJ, Wu HH, Li QH (2014) Mechanochemical milling promoted solvent-free imino Diels–Alder reaction catalyzed by FeCl3: diastereoselective synthesis of cis-2, 4-diphenyl-1,2,3,4-tetrahydroquinolines. RSC Adv 4(67):35635–35638. https://doi.org/10.1039/C4RA05252H
Janković N, Stefanović S, Petronijević J, Joksimović N, Novaković SB, Bogdanović GA, Muškinja J, Vraneš M, Ratković Z, Bugarčić Z (2018) Water-tuned tautomer-selective tandem synthesis of the 5, 6-dihydropyrimidin-4(3H)-ones, driven under the umbrella of sustainable chemistry. ACS Sustain Chem Eng 6(10):13358–13366. https://doi.org/10.1021/acssuschemeng.8b03127
Valdez-Camacho JR, Cortés-Guzmán KP, Torres-Gómez H, Flores R, Leyva MA, Escalante J (2019) Kinetics, thermodynamics, and theoretical studies in a Diels–Alder dimerization process of 3-vinylindole derivative of the 3-indoleacetic acid: an auxin. ChemistrySelect 4(28):8311–8316. https://doi.org/10.1002/slct.201901141
Long J, Hu J, Shen X, Ji B, Ding K (2002) Discovery of exceptionally efficient catalysts for solvent-free enantioselective hetero-Diels−Alder reaction. J Am Chem Soc 124(1):10–11. https://doi.org/10.1021/ja0172518
Kojima T, Inukai T (1970) Aluminum chloride catalyzed diene condensation. V. Selectivity-reactivity relation of dienophiles toward butadiene, isoprene, and 2-trifluoromethyl butadiene. J Org Chem 35(5):1342–1348. https://doi.org/10.1021/jo00830a019
Fringuelli F, Girotti R, Pizzo F, Vaccaro L (2006) [AlCl3+ 2THF]: a new and efficient catalytic system for Diels–Alder cycloaddition of α, β-unsaturated carbonyl compounds under solvent-free conditions. Org Lett 8(12):2487–2489. https://doi.org/10.1021/ol060569q
Merchan Arenas DR, Kouznetsov VV (2014) Diastereoselective synthesis of dihydroisoindolo [2,1-a] quinolin-11-ones by solvent-free AMCell-SO3H-catalyzed imino Diels–Alder/intramolecular amide cyclization cascade reactions. J Org Chem 79(11):5327–5333. https://doi.org/10.1021/jo500516c
Díaz-Ortiz Á, Prieto P, De La Hoz A (2019) A critical overview on the effect of microwave irradiation in organic synthesis. Chem Rec 19(1):85–97. https://doi.org/10.1002/tcr.201800059
De la Hoz A, Díaz-Ortiz A, Prieto P (2016) Microwave-assisted green organic synthesis. CHAPTER 1. In: Stefanidis G, Stankiewicz A (eds) Alternative energy sources for green chemistry. Royal Society of Chemistry, London, pp 1–33. https://doi.org/10.1039/9781782623632-00001
Sarma R, Sarmah MM, Prajapati D (2012) Microwave-promoted catalyst-and solvent-free aza-Diels–Alder reaction of aldimines with 6-[2-(dimethylamino) vinyl]-1, 3-dimethyluracil. J Org Chem 77(4):2018–2023. https://doi.org/10.1021/jo202346w
Flores-Conde MI, Reyes L, Herrera R, Rios H, Vazquez MA, Miranda R, Tamariz J, Delgado F (2012) Highly regio-and stereoselective Diels–Alder cycloadditions via two-step and multicomponent reactions promoted by infrared irradiation under solvent-free conditions. Int J Mol Sci 13(3):2590–2617. https://doi.org/10.3390/ijms13032590
Naskar S, Roy Chowdhury S, Mondal S, Maiti DK, Mishra S, Das I (2019) Visible-light-activated divergent reactivity of dienones: dimerization in neat conditions and regioselective E to Z isomerization in the solvent. Org Lett 21(6):1578–1582. https://doi.org/10.1021/acs.orglett.9b00083
Kumamoto K, Fukada I, Kotsuki H (2004) Diels–Alder reaction of thiophene: dramatic effects of high-pressure/solvent-free conditions. Angew Chem Int Ed 43(15):2015–2017. https://doi.org/10.1002/anie.200353487
Sun D, Sato F, Yamada Y, Sato S (2013) Solvent-free Diels–Alder reaction in a closed batch system. Bull Chem Soc Jpn 86(2):276–282. https://doi.org/10.1246/bcsj.20120247
Crouillebois L, Pantaine L, Marrot J, Coeffard V, Moreau X, Greck C (2015) Solvent-and catalyst-free synthesis of nitrogen-containing bicycles through hemiaminal formation/diastereoselective hetero-Diels–Alder reaction with diazenes. J Org Chem 80(1):595–601. https://doi.org/10.1021/jo502087a
Patterson AL, May MD, Visser BJ, Kislukhin AA, Vosburg DA (2013) Solvent-free synthesis and fluorescence of a thiol-reactive sensor for undergraduate organic laboratories. J Chem Educ 90(12):1685–1687. https://doi.org/10.1021/ed400445j
Krinochkin AP, Reddy GM, Kopchuk DS, Slepukhin PA, Shtaitz YK, Khalymbadzha IA, Kovalev IS, Kim GA, Ganebnykh IN, Zyryanov GV, Chupakhin ON (2021) 2-Aminooxazoles as novel dienophiles in the inverse demand Diels–Alder reaction with 1, 2, 4-triazines. Mendeleev Commun 31(4):542–544. https://doi.org/10.1016/j.mencom.2021.07.035
Hu Y, Liu C, Wang P, Li G, Wang A, Cong Y, Liang X, Li W, Zhang X, Li N (2020) Sustainable production of safe plasticizers with bio-based fumarates and 1, 3-dienes. Ind Eng Chem Res 59(16):7367–7374. https://doi.org/10.1021/acs.iecr.9b05840
Flores-Larios IY, López-Garrido L, Martínez-Martínez FJ, González J, García-Báez EV, Cruz A, Padilla-Martínez II (2010) Thermal [4+ 2] cycloadditions of 3-acetyl-, 3-carbamoyl-, and 3-ethoxycarbonyl-coumarins with 2, 3-dimethyl-1, 3-butadiene under solventless conditions: a structural study. Molecules 15(3):1513–1530. https://doi.org/10.3390/molecules15031513
Wang T, Hoye TR (2015) Diels–Alderase-free, bis-pericyclic,[4+ 2] dimerization in the biosynthesis of (±)-paracaseolide A. Nat Chem 7(8):641–645. https://doi.org/10.1038/nchem.2281
Zentar H, Arias F, Haidour A, Alvarez-Manzaneda R, Chahboun R, Alvarez-Manzaneda E (2018) Protecting-group-free synthesis of cassane-type furan diterpenes via a decarboxylative dienone-phenol rearrangement. Org Lett 20(22):7007–7010. https://doi.org/10.1021/acs.orglett.8b02867
Quijano-Quiñones RF, Castro-Segura CS, Mena-Rejón GJ, Quesadas-Rojas M, Cáceres-Castillo D (2018) Biosynthesis of grandione: an example of tandem hetero Diels–Alder/Retro–Claisen rearrangement reaction? Molecules 23(10):2505. https://doi.org/10.3390/molecules23102505
Orzolek BJ, Rahman MA, Iovine PM (2018) Synthesis of biorenewable starch–Farnesene amphiphilic conjugates via transesterification of terpene-derived Diels–Alder adducts. ACS Sustain Chem Eng 6(10):13562–13569. https://doi.org/10.1021/acssuschemeng.8b03771
Cao Y, Osuna S, Liang Y, Haddon RC, Houk KN (2013) Diels–Alder reactions of graphene: computational predictions of products and sites of reaction. J Am Chem Soc 135(46):17643–17649. https://doi.org/10.1021/ja410225u
Oshima T, Mikie T, Ikuma N, Yakuma H (2012) First kinetic evidence for the CH/π and π/π solute–solvent interaction of C60 in the Diels–Alder reaction with cyclohexadiene. Org Biomol Chem 10(9):1730–1734. https://doi.org/10.1039/C2OB06748J
Kalaoglu-Altan OI, Sanyal R, Sanyal A (2015) “Clickable” polymeric nanofibers through hydrophilic–hydrophobic balance: fabrication of robust biomolecular immobilization platforms. Biomacromol 16(5):1590–1597. https://doi.org/10.1021/acs.biomac.5b00159
Oh CR, Lee DI, Park JH, Lee DS (2019) Thermally healable and recyclable graphene-nanoplate/epoxy composites via an in-situ Diels–Alder reaction on the graphene-nanoplate surface. Polymers 11(6):1057. https://doi.org/10.3390/polym11061057
Oh CR, Lee SH, Park JH, Lee DS (2019) Thermally self-healing graphene-nanoplate/polyurethane nanocomposites via diels–alder reaction through a one-shot process. Nanomaterials 9(3):434. https://doi.org/10.3390/nano9030434
Seo JM, Baek JB (2014) A solvent-free Diels–Alder reaction of graphite into functionalized graphene nanosheets. Chem Commun 50(93):14651–14653. https://doi.org/10.1039/C4CC07173E
Yamada H, Ohkubo K, Kuzuhara D, Takahashi T, Sandanayaka AS, Okujima T, Ohara K, Ito O, Uno H, Ono N, Fukuzumi S (2010) Synthesis, crystal structure, and photodynamics of π-expanded porphyrin− fullerene dyads synthesized by Diels−Alder reaction. J Phy Chem B 114(45):14717–14728. https://doi.org/10.1021/jp102966x
Cao R, Wang Y, Chen S, Han N, Liu H, Zhang X (2019) Multiresponsive shape-stabilized hexadecyl acrylate-grafted graphene as a phase change material with enhanced thermal and electrical conductivities. ACS Appl Mater Interfaces 11(9):8982–8991. https://doi.org/10.1021/acsami.8b18282
Acknowledgements
This work was financially supported by the Grants Council of the President of the Russian Federation (# NSh-1223.2022.1.3) and Russian Scientific Foundation (Grant # 21-13-00304).
Author information
Authors and Affiliations
Contributions
The authors AR and APK conceived the study and collected the literature associated with the review. AR, APK, and AFK performed the manuscript writing and analysis of the data. DSK and GVZ supported with their attentive discussions and advice to fulfill this study. The final version of the manuscript was submitted after it had been read and approved by all authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests related to this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rammohan, A., Krinochkin, A.P., Khasanov, A.F. et al. Sustainable Solvent-Free Diels–Alder Approaches in the Development of Constructive Heterocycles and Functionalized Materials: A Review. Top Curr Chem (Z) 380, 43 (2022). https://doi.org/10.1007/s41061-022-00398-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41061-022-00398-2