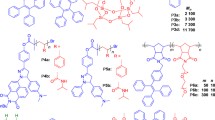
Abstract
Clusteroluminescence is a phenomenon whereby the aggregation or clustering of non-conjugated electron-rich units leads to the emission of light at long wavelengths. This phenomenon was first discovered in poly(amido amine) (PAMAM) dendrimers. In recent years, clusteroluminescence has attracted growing research interest and its photophysical properties and mechanism have been thoroughly studied. In this review, we first briefly introduce the development of different types of clusteroluminogens. Then we highlight recent developments in clusteroluminescence, including mechanistic studies, the disclosure of room-temperature phosphorescence, and the extension of emission to the longer-wavelength region. Lastly, we demonstrate a few applications in various fields. With advantages such as being earth-abundant, biocompatible and biodegradable, clusteroluminogens are envisioned to be commonplace in the future.











Similar content being viewed by others
References
Stokes GG (1854) On the change of refrangibility of light. Abstr Pap Commun R Soc Lond 6:195–200
Luo J, Xie Z, Lam JWY, Cheng L, Tang BZ, Chen H, Qiu C, Kwok HS, Zhan X, Liu Y, Zhu D (2001) Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem Commun 18:1740–1741
Zhang H, Zhao Z, Turley AT, Wang L, McGonigal PR, Tu Y, Li Y, Wang Z, Kwok RTK, Lam JWY, Tang BZ (2020) Aggregate science: from structures to properties. Adv Mater 32(36):2001457
Mei J, Leung NLC, Kwok RTK, Lam JWY, Tang BZ (2015) Aggregation-induced emission: together we shine, united we soar! Chem Rev 115(21):11718–11940
Tomalia DA, Klajnert-Maculewicz B, Johnson KAM, Brinkman HF, Janaszewska A, Hedstrand DM (2019) Non-traditional intrinsic luminescence: inexplicable blue fluorescence observed for dendrimers, macromolecules and small molecular structures lacking traditional/conventional luminophores. Prog Polym Sci 90:35–117
Zhang H, Zhao Z, McGonigal PR, Ye R, Liu S, Lam JWY, Kwok RTK, Yuan WZ, Xie J, Rogach AL, Tang BZ (2020) Clusterization-triggered emission: uncommon luminescence from common materials. Mater Today 32:275–292
He B, Zhang J, Zhang J, Zhang H (2020) Clusteroluminescence from cluster excitons in small heterocyclics free of aromatic rings. ChemRxiv. https://doi.org/10.26434/chemrxiv.13200491.v1
Wang Y, Zhao Z, Yuan WZ (2020) Intrinsic luminescence from nonaromatic biomolecules. ChemPlusChem 85(5):1065–1080
Halpern AM (1970) The vapor state emission from a saturated amine. Chem Phys Lett 6(4):296–298
Fan Y, Fan Y, Wang Y, Ma J (2007) Unexpected fluorescence emission of poly(α, β-L-malic acid) in aqueous medium. J Appl Polym Sci 106(3):1640–1647
Wang D, Imae T (2004) Fluorescence emission from dendrimers and its PH dependence. J Am Chem Soc 126(41):13204–13205
Lee WI, Bae Y, Bard AJ (2004) Strong blue photoluminescence and ECL from OH-terminated PAMAM dendrimers in the absence of gold nanoparticles. J Am Chem Soc 126(27):8358–8359
Larson CL, Tucker SA (2001) Intrinsic fluorescence of carboxylate-terminated polyamido amine dendrimers. Appl Spectrosc 55(6):679–683
Zhen S, Mao J-C, Chen L, Ding S, Luo W, Zhou X-S, Qin A, Zhao Z, Tang BZ (2018) Remarkable multichannel conductance of novel single-molecule wires built on through-space conjugated hexaphenylbenzene. Nano Lett 18(7):4200–4205
Joo Y, Agarkar V, Sung SH, Savoie BM, Boudouris BW, Zhang H, Zheng X, Kwok RTK, Wang J, Leung NLC, Shi L, Sun JZ, Tang Z, Lam JWY, Qin A, Tang BZ (2018) A nonconjugated radical polymer glass with high electrical conductivity. Science 359:1391–1395
Nordlander P, Oubre C, Prodan E, Li K, Stockman MI (2004) Plasmon hybridization in nanoparticle dimers. Nano Lett 4(5):899–903
Prodan E, Radloff C, Halas NJ, Nordlander P (2003) A hybridization model for the plasmon response of complex nanostructures. Science 302:419–422
Yanari SS, Bovey FA, Lumry R (1963) Fluorescence of styrene homopolymers and copolymers. Nature 200(4903):242–244
Vallan L, Urriolabeitia EP, Ruipérez F, Matxain JM, Canton-Vitoria R, Tagmatarchis N, Benito AM, Maser WK (2018) Supramolecular-enhanced charge transfer within entangled polyamide chains as the origin of the universal blue fluorescence of polymer carbon dots. J Am Chem Soc 140(40):12862–12869
Zheng J, Petty JT, Dickson RM (2003) High quantum yield blue emission from water-soluble Au8 nanodots. J Am Chem Soc 125(26):7780–7781
Cao L, Yang W, Wang C, Fu S (2007) Synthesis and striking fluorescence properties of hyperbranched poly(amido amine). J Macromol Sci Part A Pure Appl Chem 44(4):417–424
Wang D, Imae T, Miki M (2007) Fluorescence emission from PAMAM and PPI dendrimers. J Colloid Interface Sci 306(2):222–227
Wu D, Liu Y, He C, Goh SH (2005) Blue photoluminescence from hyperbranched poly(amino ester)s. Macromolecules 38(24):9906–9909
Fernández L, Sigal E, Otero L, Silber JJ, Santo M (2011) Solubility improvement of an anthelmintic benzimidazole carbamate by association with dendrimers. Braz J Chem Eng 28:679–689
Homchaudhuri L, Swaminathan R (2001) Novel absorption and fluorescence characteristics of l-lysine. Chem Lett 8:844–845
Al-Jamal KT, Ruenraroengsak P, Hartell N, Florence AT (2006) An intrinsically fluorescent dendrimer as a nanoprobe of cell transport. J Drug Target 14(6):405–412
Ellis B, Brignola P, Brashear RL, Thomas R, Dickerson SH, Dickson HD, Kelly H, Gaul M, Griffin RJ, Hassell AM, Keith B, Mullin R, Petrov KG, Reno MJ, Rusnak DW, Tadepalli SM, Ulrich JC, Craig D, Vanderwall DE, Waterson AG, Williams JD, White WL, Uehling DE, Pompa PP, Maruccio G, Della A, Sabella S, Tamburro AM, Laureana L, Pompa PP, Maruccio G, Torre X, Della A, Sabella S, Tamburro AM, Cingolani R, Rinaldi R (2007) Charge transport and intrinsic fluorescence in amyloid-like fibrils. Proc Natl Acad Sci USA 104(46):18019–18024
Ye R, Liu Y, Zhang H, Su H, Zhang Y, Xu L, Hu R, Kwok RTK, Wong KS, Lam JWY, Goddard WA, Tang BZ (2017) Non-conventional fluorescent biogenic and synthetic polymers without aromatic rings. Polym Chem 8(10):1722–1727
Caminade AM, Yan D, Smith DK (2015) Dendrimers and hyperbranched polymers. Chem Soc Rev 44:3870–3873
Sun M, Hong CY, Pan CY (2012) A unique aliphatic tertiary amine chromophore: fluorescence, polymer structure, and application in cell imaging. J Am Chem Soc 134(51):20581–20584
Restani RB, Morgado PI, Ribeiro MP, Correia IJ, Aguiar-Ricardo A, Bonifácio VDB (2012) Biocompatible polyurea dendrimers with PH-dependent fluorescence. Angew Chem 124(21):5252–5255
Lu H, Feng L, Li S, Zhang J, Lu H, Feng S (2015) Unexpected strong blue photoluminescence produced from the aggregation of unconventional chromophores in novel siloxane-poly(amidoamine) dendrimers. Macromolecules 48(3):476–482
Pastor-Pérez L, Chen Y, Shen Z, Lahoz A, Stiriba SE (2007) Unprecedented blue intrinsic photoluminescence from hyperbranched and linear polyethylenimines: polymer architectures and PH-effects. Macromol Rapid Commun 28(13):1404–1409
Wang R, Yuan W, Zhu X (2015) Aggregation-induced emission of non-conjugated poly(amido amine)s: discovering, luminescent mechanism understanding and bioapplication. Chin J Polym Sci (English Ed) 33(5):680–687
Zhou Q, Cao B, Zhu C, Xu S, Gong Y, Yuan WZ, Zhang Y (2016) Clustering-triggered emission of nonconjugated polyacrylonitrile. Small 12(47):6586–6592
Wang Y, Bin X, Chen X, Zheng S, Zhang Y, Yuan WZ (2018) Emission and emissive mechanism of nonaromatic oxygen clusters. Macromol Rapid Commun 39(21):1–6
Lin SY, Wu TH, Jao YC, Liu CP, Lin HY, Lo LW, Yang CS (2011) Unraveling the photoluminescence puzzle of PAMAM dendrimers. Chem A Eur J 17(26):7158–7161. https://doi.org/10.1002/chem.201100620
Zhang Z, Li D, Jiang W, Wang Z (2018) The electron density delocalization of hydrogen bond systems. Adv Phys X 3(1):298–315
Wang B, Xin M, Dai X, Song R, Meng Y, Han J, Jiang W, Wang Z, Zhang R (2015) Electronic delocalization in small water rings. Phys Chem Chem Phys 17(5):2987–2990
Wang L, Fried SD, Boxer SG, Markland TE (2014) Quantum delocalization of protons in the hydrogen-bond network of an enzyme active site. Proc Natl Acad Sci USA 111(52):18454–18459
Grisanti L, Pinotsi D, Gebauer R, Kaminski Schierle GS, Hassanali AA (2017) A computational study on how structure influences the optical properties in model crystal structures of amyloid fibrils. Phys Chem Chem Phys 19(5):4030–4040
Pinotsi D, Grisanti L, Mahou P, Gebauer R, Kaminski CF, Hassanali A, Kaminski Schierle GS (2016) Proton transfer and structure-specific fluorescence in hydrogen bond-rich protein structures. J Am Chem Soc 138(9):3046–3057
Zhao Z, Zhang H, Lam JWY, Tang BZ (2020) Aggregation-induced emission: new vistas at the aggregate level. Angew Chem 20:20
Wang Q, Dou X, Chen X, Zhao Z, Wang S, Wang Y, Sui K, Tan Y, Gong Y, Zhang Y, Yuan WZ (2019) Reevaluating protein photoluminescence: remarkable visible luminescence upon concentration and insight into the emission mechanism. Angew Chem Int Ed 58(36):12667–12673
Dou X, Zhou Q, Chen X, Tan Y, He X, Lu P, Sui K, Tang BZ, Zhang Y, Yuan WZ (2018) Clustering-triggered emission and persistent room temperature phosphorescence of sodium alginate. Biomacromol 19(6):2014–2022
Zhou Q, Wang Z, Dou X, Wang Y, Liu S, Zhang Y, Yuan WZ (2019) Emission mechanism understanding and tunable persistent room temperature phosphorescence of amorphous nonaromatic polymers. Mater Chem Front 3(2):257–264
Chen X, Liu X, Lei J, Xu L, Zhao Z, Kausar F, Xie X, Zhu X, Zhang Y, Yuan WZ (2018) Synthesis, clustering-triggered emission, explosive detection and cell imaging of nonaromatic polyurethanes. Mol Syst Des Eng 3(2):364–375
Zhao Z, Chen X, Wang Q, Yang T, Zhang Y, Yuan WZ (2019) Sulphur-containing nonaromatic polymers: clustering-triggered emission and luminescence regulation by oxidation. Polym Chem 10(26):3639–3646
Eftink MR, Selva TJ, Wasylewski Z (1987) Studies of the efficiency and mechanism of fluorescence quenching reactions using acrylamide and succinimide as quenchers. Photochem Photobiol 46(1):23–30
Yan J, Zheng B, Pan D, Yang R, Xu Y, Wang L, Yang M (2015) Unexpected fluorescence from polymers containing dithio/amino-succinimides. Polym Chem 6(34):6133–6139
Wang Y, Tang S, Wen Y, Zheng S, Yang B, Yuan WZ (2020) Nonconventional luminophores with unprecedented efficiencies and color-tunable afterglows. Mater Horizons 7(8):2105–2112
Zhao E, Lam JWY, Meng L, Hong Y, Deng H, Bai G, Huang X, Hao J, Tang BZ (2015) Poly[(maleic anhydride)-alt-(vinyl acetate)]: a pure oxygenic nonconjugated macromolecule with strong light emission and solvatochromic effect. Macromolecules 48(1):64–71
Hu C, Guo Z, Ru Y, Song W, Liu Z, Zhang X, Qiao J (2018) A new family of photoluminescent polymers with dual chromophores. Macromol Rapid Commun 39(10):1–6
Zhou Q, Yang T, Zhong Z, Kausar F, Wang Z, Zhang Y, Yuan WZ (2020) A Clustering-triggered emission strategy for tunable multicolor persistent phosphorescence. Chem Sci 11(11):2926–2933
Teki Y (2020) Excited-state dynamics of non-luminescent and luminescent π-radicals. Chem A Eur J 26(5):980–996
Wang Z, Zou X, Xie Y, Zhang H, Hu L, Chan CCS (2020) A nonconjugated radical polymer with stable red luminescence in solid state. ChemRxiv. https://doi.org/10.26434/chemrxiv.12924200.v1
Hirayama F (1965) Intramolecular excimer formation. I. Diphenyl and triphenyl alkanes. J Chem Phys 42(9):3163–3171
Brown CJ, Farthing AC (1949) Preparation and structure of di-p-xylylene [2]. Nature 20:915–916
Zhang H, Zheng X, Xie N, He Z, Liu J, Leung NLC, Niu Y, Huang X, Wong KS, Kwok RTK, Sung HHY, Williams ID, Qin A, Lam JWY, Tang BZ (2017) Why do simple molecules with “isolated” phenyl rings emit visible light? J Am Chem Soc 139(45):16264–16272
Han T, Deng H, Qiu Z, Zhao Z, Zhang H, Zou H, Leung NLC, Shan G, Elsegood MRJ, Lam JWY, Tang BZ (2018) Facile multicomponent polymerizations toward unconventional luminescent polymers with readily openable small heterocycles. J Am Chem Soc 140(16):5588–5598
Chen L, Wang YH, He B, Nie H, Hu R, Huang F, Qin A, Zhou XS, Zhao Z, Tang BZ (2015) Multichannel conductance of folded single-molecule wires aided by through-space conjugation. Angew Chem Int Ed 54(14):4231–4235
Robb MJ, Li W, Gergely RCR, Matthews CC, White SR, Sottos NR, Moore JS (2016) A robust damage-reporting strategy for polymeric materials enabled by aggregation-induced emission. ACS Cent Sci 2(9):598–603
Bartholomew GP, Bazan GC (2001) Bichromophoric paracyclophanes: models for interchromophore delocalization. Acc Chem Res 34(1):30–39
Zhang H, Du L, Wang L, Liu J, Wan Q, Kwok RTK, Lam JWY, Phillips DL, Tang BZ (2019) Visualization and manipulation of molecular motion in the solid state through photoinduced clusteroluminescence. J Phys Chem Lett 10(22):7077–7085
Yang W, Pan CY, Luo MD, Bin ZH (2010) Fluorescent mannose-functionalized hyperbranched poly(amido amine)s: synthesis and interaction with E. coli. Biomacromol 11(7):1840–1846
Yang W, Pan CY, Liu XQ, Wang J (2011) Multiple functional hyperbranched poly(amido amine) nanoparticles: synthesis and application in cell imaging. Biomacromol 12(5):1523–1531
Qiu L, Zhu C, Chen H, Hu M, He W, Guo Z (2014) A turn-on fluorescent Fe3+ sensor derived from an anthracene-bearing bisdiene macrocycle and its intracellular imaging application. Chem Commun 50(35):4631–4634
Tao S, Lu S, Geng Y, Zhu S, Redfern SAT, Song Y, Feng T, Xu W, Yang B (2018) Design of metal-free polymer carbon dots: a new class of room-temperature phosphorescent materials. Angew Chem Int Ed 57:2393–2398
Zhen S, Mao JC, Chen L, Ding S, Luo W, Zhou XS, Qin A, Zhao Z, Tang BZ (2018) Remarkable multichannel conductance of novel single-molecule wires built on through-space conjugated hexaphenylbenzene. Nano Lett 18:4200–4205
Li J, Shen P, Zhao Z, Tang BZ (2019) Through-space conjugation: a thriving alternative for optoelectronic materials. CCS Chem 1(2):181–196
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Z., Zhang, H., Li, S. et al. Recent Advances in Clusteroluminescence. Top Curr Chem (Z) 379, 14 (2021). https://doi.org/10.1007/s41061-021-00326-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41061-021-00326-w