Abstract
Patients with coronavirus disease 2019 (COVID-19) usually suffer from post-acute sequelae of coronavirus disease 2019 (PASC). Pulmonary fibrosis (PF) has the most significant long-term impact on patients’ respiratory health, called post-COVID-19 pulmonary fibrosis (PC19-PF). PC19- PF can be caused by acute respiratory distress syndrome (ARDS) or pneumonia due to COVID-19. The risk factors of PC19-PF, such as older age, chronic comorbidities, the use of mechanical ventilation during the acute phase, and female sex, should be considered. Individuals with COVID-19 pneumonia symptoms lasting at least 12 weeks following diagnosis, including cough, dyspnea, exertional dyspnea, and poor saturation, accounted for nearly all disease occurrences. PC19-PF is characterized by persistent fibrotic tomographic sequelae associated with functional impairment throughout follow-up. Thus, clinical examination, radiology, pulmonary function tests, and pathological findings should be done to diagnose PC19-PF patients. PFT indicated persistent limitations in diffusion capacity and restrictive physiology, despite the absence of previous testing and inconsistency in the timeliness of assessments following acute illness. It has been hypothesized that PC19-PF patients may benefit from idiopathic pulmonary fibrosis treatment to prevent continued infection-related disorders, enhance the healing phase, and manage fibroproliferative processes. Immunomodulatory agents might reduce inflammation and the length of mechanical ventilation during the acute phase of COVID-19 infection, and the risk of the PC19-PF stage. Pulmonary rehabilitation, incorporating exercise training, physical education, and behavioral modifications, can improve the physical and psychological conditions of patients with PC19-PF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Patients with COVID-19 usually suffer from post-acute sequelae of coronavirus disease 2019 (PASC). |
Pulmonary fibrosis (PF) is the most significant long-term effect on patients’ respiratory health. |
Risk factors for PASC-induced pulmonary fibrosis include advanced age, chronic comorbidities, the use of mechanical ventilation during the acute phase, and female patients. |
The diagnosis of pulmonary fibrosis post-COVID should be based on clinical examination, radiological features, pulmonary function tests, and pathological biomarkers. |
The use of antifibrotic treatment for patients with acute respiratory distress syndrome or pneumonia due to COVID-19 might prevent the fibroproliferative process. |
Future research is required to have a better understanding of the natural history of pulmonary fibrosis post-COVID, as well as its pathogenesis and clinical and psychosocial impact on patients |
Introduction
The majority of survivors of COVID-19 suffer from post-acute complications. Accumulating evidence indicates that COVID-19 has subacute and long-term effects. The most long-term respiratory morbidity and impact on patients’ respiratory health is post-COVID-19 pulmonary fibrosis (PC19-PF) [1]. PC19-PF can be caused by acute respiratory distress syndrome (ARDS) and pneumonia at acute COVID-19 infection [2, 3]. Moreover, other risk factors of respiratory PASC can be considered, such as age, chronic comorbid diseases, mechanical ventilation, and the female sex [2, 4]. Several studies mention screening patients at risk for PC19-PF with pulmonary function testing (PFT) and cross-sectional chest computed tomography (CT) scan to evaluate the consequences for future considerations and treatments [5,6,7,8,9].
In this review, we tried to determine the probability risk that COVID-19 infection poses on the development of PF, considering the critical postinfection outcomes. This review also tried to assess the PF severity, the management, and follow-up on the progression of PF for selecting the specific treatments, which focus on the patient’s phenotype and their pathological, radiological, and clinical features.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Definition, Patient Demographics, and Risk Factors for Post-COVID-19 Pulmonary Fibrosis
Definition of Post-COVID-19 Pulmonary Fibrosis
PC19-PF is a lung disorder that does not yet have a well-described particular definition, prevalence, pathophysiology, or treatment. According to a systematic review, PC19-PF was observed in 7.0% of patients across the five included studies [9]. According to the findings of another study, 40% of COVID-19 patients will develop ARDS, and 20% of these instances will be severe [10]. It will take some time for the prevalence of PC19-PF to become apparent; nevertheless, preliminary research carried on patients with COVID-19 who had just been discharged from the hospital reveals that more than a third of individuals who recover go on to acquire fibrotic abnormalities [11].
Post-COVID-19 pulmonary fibrosis (PC19-PF) is the existence of persistent fibrotic tomographic sequelae linked with functional impairment during follow-up [12]. However, more information must be given regarding the disorder’s prevalence, pathogenesis, probable risk factors, and potential therapeutic approach. The following criteria are used to classify post-COVID-19 patients: (1) persistent symptoms following an acute phase or the onset of new symptoms, (2) deterioration, loss of quality of life, and functional state compared with before COVID-19, and (3) radiological abnormalities that are constant or getting worse, and abnormal findings in the lungs [13].
There are misunderstandings in particular conditions that make defining post-COVID-19 difficult. It is difficult to establish a timeline for acute and chronic symptoms, especially when the same symptoms appear in both periods. Second, post-COVID-19 complications are challenging to separate from postintensive care difficulties (malnutrition, immobility, anxiety, etc.), post-ARDS, postmechanical ventilation (barotrauma, fibrosis, pneumothorax, etc.) complications, and postintubation complications (tracheal trauma, edema, stenosis, etc.). Finally, to distinguish between past comorbidities and COVID-19 effects, the patient’s pre-COVID-19 baseline status is required.
It is essential to determine whether the clinical outcome is caused by post-COVID-19 or a secondary infection. Furthermore, common problems associated with corticosteroid use, postimmunosuppression effects, post-traumatic syndrome, post-thrombotic effects, and postischemic effects should be considered [14].
It is unknown what the features are of individuals admitted to the hospital with respiratory problems and radiologic lung abnormalities after the administration of COVID-19, particularly concerning the progression of PC19-PF. Clinical examination, chest radiology, pulmonary function tests, and pathological findings should be done to diagnose PC19-PF patients. Even though no one knows when the best time is to identify irreversible PF, many experts suggest doing lung function tests, chest CT scans, and exercise tests 3, 6, and 12 months after an acute COVID-19 episode [12].
Patient Demographics and Risk Factors for Post-COVID-19 Pulmonary Fibrosis
Patients diagnosed with COVID-19 pneumonia whose cough, dyspnea, exertional dyspnea, and poor saturation continued for at least 12 weeks after the diagnosis made up almost all of the cases. Some individuals with new-onset dyspnea had a score on the Nijmegen questionnaire that was higher than 22, indicating “functional respiratory complaints.” In contrast, others had fibrotic lesions with lower respiratory volumes on pulmonary function tests. Patients with new-onset dyspnea had diminished forced vital capacity (FVC) and total lung capacity (TLC), suggesting a possible role for lung sequelae in this condition. In addition to respiratory consequences, other potential causes of dyspnea include dysfunction of the circulatory system and deconditioning of the muscles [15, 16].
Potential risk factors for PC19-PF survivors can be divided into two categories: patient-related and disease-related [16] (Fig. 1). According to some studies, patient-related risk factors for PF include advanced age, male gender, active smoking, a history of chronic alcoholism, and having underlying disorders such as diabetes and respiratory or cardiovascular diseases. Prolonged ICU stay and mechanical ventilation duration, the use of HFNC (high flow nasal cannula), the presence of ARDS, and the degree of systemic inflammation have also been linked to an increased risk of PF [17] (Fig. 1). Furthermore, high CRP (C-reactive protein), IL (interleukin)-6, and LDH (lactate dehydrogenase) levels in the acute phase may activate fibroblast proliferation in the lung injury repair process [12] (Fig. 1).
Pathophysiological Mechanisms of Post-COVID Pulmonary Fibrosis
Two years into the COVID-19 pandemic, there is mounting evidence demonstrating that many COVID-19 patients develop fibrotic sequelae and changes in lung function in post-COVID stage. It suggests that restrictive lung disease is a hallmark PC19-PF patients. Thus, PF has been considered as one of the complications of severe COVID-19 infection seen in the third stage of COVID-19 patients. Severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) are the examples of mild to severe illnesses. In addition, ARDS is a major complication usually seen in critically infected patients with SARS coronavirus 2 (SARS-CoV-2) [1]. However, the pathology of acute COVID-19 and PC19-PF, on the other hand, is unclear [3]. PF can develop as a result of poor lung injury resolution or an exaggeration of the healing process [19]. ARDS can be caused by a variety of pulmonary or extrapulmonary insults, but there may be a common downstream pathway leading to fibrosis. This pathway could be aggravated by cytokine production by lung epithelial cells and macrophages, or it could be caused by harmful mechanical ventilation [20].
The nasal epithelial cell area has an important role in SARS-CoV-2 (COVID-19) infection because it is the main way for COVID-19 leading to the respiratory system. In fact, COVID-19 enters the respiratory tract through ACE-2 receptors on the pulmonary epithelium. The virus can infiltrate the lower respiratory tract and infect type-II alveolar cells in the lungs, causing diffuse alveolar damage (DAD) [1] (Fig. 2). Upregulation of MMP2, MMP8, and cathepsin proteins, and downregulation of E-cadherin protein, may also result in PF. Proteins like laminins, collagen VI, annexin A2, and fibronectin, which are the components of the extracellular matrix (ECM) of the basement membrane of the lung, are also downregulated [21]. TGF-β, a major profibrotic stimulus, is directly amplified by the SARS-CoV-1 nucleocapsid protein. Because the nucleocapsid proteins of SARS-CoV-2 are 90% identical to those of SARS-CoV-1, it can be hypothesized that it is one of the possible mechanisms for lung fibrosis. TGF- β, like connective tissue growth factor, is upregulated by angiotensin II, which accumulates in the lungs due to the virus’s downregulation of ACE-2 [22].
Whether post-COVID-19 fibrotic lung alterations are stable or progressing, like in fibrotic lung illnesses such idiopathic pulmonary fibrosis (IPF), it is important to ascertain for patient management and treatment. There are many factors that could influence whether PC19-PF progresses and becomes life-threatening. In genetic studies of COVID-19, genes involved in innate antiviral defenses, inflammatory lung injury, and the ABO blood-group system have all been linked to life-threatening diseases. Prospective genome-wide studies of COVID-19-related fibrotic lung will illuminate genetics’ role in progressive or stable fibrosis. Age is a major risk factor for both lung fibrosis and COVID-19, and it may play a role in whether fibrosis progresses. It is associated with lung parenchymal stiffening, which may have significant effects for TGF activation and the development of lung fibrosis. The profibrotic potential of lung fibroblasts is also affected by age. Moreover, obesity and metabolic syndrome are common COVID-19 risk factors. Diabetes, both type 1 and type 2, is also associated with a remarkably increased risk of death from COVID-19 [23].
Screening for Patients with Post-COVID-19-Pulmonary Fibrosis
Pulmonary Function Testing (PFT)
Different challenges in the diagnosis of PC19-PF must be thoroughly investigated as soon as possible. During the COVID-19 pandemic outbreak, PFT demonstrated chronic limitations in diffusion capacity and restrictive physiology by the lack of baseline testing and nonuniformity in timing, from acute illness or follow-up assessment [3]. PFT also revealed that these limitations were present despite baseline testing not being done. The British Thoracic Society recommends that all survivors of critical COVID-19 should undergo PFTs every three months after discharge, and those with mild to moderate disease should experience PFTs if radiographic imaging is aberrant [24]. A recent meta-analysis discovered that 39% of COVID-19 survivors had decreased lung diffusing capacity, 15% of the individuals had a restrictive pattern of ventilation, while 7% had an obstructive type of ventilation [25]. As a consequence of this, we propose that testing for lung function should be carried out on all patients with COVID-19 who present themselves for treatment for PASC at any stage.
Radiographic Features
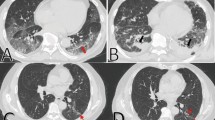
The second tool for determining throughout the pandemic, radiographic features of acute COVID-19 infection have been a major focus of research. In a large cohort of patients from Wuhan, China, over half of those who had CT scans 6 months after admission had abnormal radiographic findings. The most common findings were pulmonary interstitial changes, which were reported as ground-glass opacities and irregular lines [25] (Fig. 3). Solomon et al. [26] concluded that CT appearances in PASC should be standardised as predominantly ground glass, predominantly fibrotic, and mixed ground glass and fibrotic.
There are several unknowns about PC19-PF that should be investigated as soon as possible. First, the tomographic features of pulmonary fibrosis must be defined. Six months after diagnosis, serial tomographic assessments may reveal PC19-PF abnormalities [27]. Inpatients with COVID-19, without mechanical ventilation, found that while the majority of patients improved in tomography, pulmonary function, and exercise-related variables, 24% of those still had abnormalities on CT scans 1 year after discharge [27, 28]. Patients experiencing significant acute symptoms of COVID-19 typically have radiographic indications of fibrosis, bronchial dilatation, parenchymal bands, and coarse subpleural reticulation that does not include honeycombing [29].
Investigations have found that 38% of patients had respiratory chest radiographic abnormalities at a median of 54 days post-discharge, and 9% of individuals studied had deteriorating radiographic abnormalities during the course of the study’s follow-up [30]. Second, an elevated risk of developing PC19-PF may be defined by the existence of genetic traits, as well as past interstitial lung abnormalities, in addition to the hypotheses of autoimmune inflammatory activity that is created by viral infection.
A recent study found that patients 4 months following COVID-19 treatment were more likely to have fibrotic-like tomographic abnormalities if their blood leukocyte telomere length was shorter, lending credence to the hypothesis of genetic susceptibility to PC19-PF [31]. Furthermore, additional research evaluating the histological characteristics of PC19-PF patients is required to acquire a better understanding of this entity [32]. D’Cruz et al. emphasized the possibility that ongoing or deteriorating radiographic abnormalities may be overlooked on simple films in comparison with cross-sectional imaging. Patients with COVID-19-related lung illness were only discovered in 13% of the study population, although 46% of participants reported increased dyspnea after infection, and 75% of patients who had CT scans were diagnosed with interstitial lung disease and/or airways disease [33].
Patients with chronic dyspnea following acute COVID-19 are now screened for pulmonary fibrosis using a combination of PFT and cross-sectional imaging. Additionally, additional studies evaluating histological features obtained from patients with PC19-PF are warranted for greater understanding of this entity [33].
Biomarkers Associated with Post-COVID 19 Pulmonary Fibrosis
Until now, there have been a few studies that have established a connection between pulmonary fibrosis and damage to alveolar epithelial cells, fibroproliferation, and matrix remodeling biomarkers. Among these, serum alveolar epithelial cell damage biomarkers like Krebs von den Lungen-6 (KL-6) show promise in predicting a higher risk of PC19-PF, but more research is needed [34]. KL-6 is a high molecular weight (200 kDa) glycoprotein classified as a human transmembrane mucin 1 (MUC1) with surface expression on type II pneumocytes; the destruction and regeneration of the air-blood barrier results in elevated serum concentrations of this clinically important biomarker [35]. Patients with severe PC19-PF had higher serum KL-6 concentrations at the time of discharge, which may have clinical significance in predicting fibrotic lung involvement [36].
Matrix Metalloproteinases 1 and 7 (MMP1 and MMP7) degrade the ECM and play an important role in pulmonary fibrosis. Proinflammatory cytokines may increase MMP expression, and thus stimulate airway remodeling [37].
Treatments of Post-COVID Pulmonary Fibrosis
It is unclear whether or which patients may benefit from the use of therapeutic modalities, such as medications and pulmonary rehabilitation, to ameliorate the impairment caused by PC19-PF. This is a major limitation in the field.
Role of Antifibrotic Medications
In the COVID-19 pandemic, acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) typically resolves to normal lung function. Yet, some cases have proceeded to the more severe critical stage of pulmonary fibrosis, generally referred to as PC19-PF, which requires immediate attention and careful therapy. It is anticipated that IPF therapy could be beneficial in the treatment of COVID-19 pneumonia, due to the similarities in the pathophysiological pathways that are involved in both IPF and COVID-19 infection. Because PC19-PF is induced by the activation of transforming growth factor beta (TGF-1), it is believed that IPF therapy could be effective in the treatment of COVID-19 pneumonia. Antifibrotic therapy is used in COVID-19 patients with the clinical goal of preventing consequences of the ongoing infection, stimulating the recovery phase, and controlling fibroproliferative processes.
There is still uncertainty about the role of antifibrotic medications, such as pirfenidone and nintedanib, in patients with persistent interstitial lung-related COVID-19 infection. These antifibrotic drugs will probably be considered for those with progressive functional impairment throughout follow-up; nevertheless, randomized controlled trials are required to react to this notion. Two antifibrotic drugs that can be used orally are commercially available to treat progressive lung fibrotic diseases, namely pirfenidone with nintedanib. The roles of antifibrotic drugs in acute COVID-19 and PASC-PF have not been figured out yet, but they are being investigated in ongoing clinical trials [3]. There is uncertain biological basis for the use of antifibrotic medicines in acute COVID-19, due to the extended infection, the predominance of early reticular alterations, and the benefit of early commencement on delayed FVC decrease [38].
Pirfenidone (PFN) is an antifibrotic drug with a considerable antiinflammatory role in treating idiopathic pulmonary fibrosis (IPF). In response to TGF-1 and other proinflammatory cytokines, PFN suppresses the accumulation and recruitment of inflammatory cells, fibroblast proliferation, and extracellular matrix deposition. In addition, PFN lowers the pathogenesis of SARS-CoV-2 by inhibiting furin (TGF-1 convertase activator), a protein effector implicated in the entrance of SARS-CoV-2 and activation of TGF-1. In addition, PFN affects signaling pathways associated with the pathogenesis of PC19-PF. The antiinflammatory and antifibrotic characteristics of PFN may diminish PC19-PF [39]. Nintedanib is another antifibrotic drug approved by the FDA for treating IPF. It is a tyrosine kinase inhibitor that inhibits fibroblast and myofibroblast cascades and may affect pulmonary angiogenesis (Figs. 1, 2 and 3).
The INPULSIS trial demonstrated that nintedanib reduces the decline of FVC in IPF, thereby slowing the progression of the disease within 4–6 weeks [40]. The US Food and Drug Administration (FDA) approved both drugs, which have distinct mechanisms of action that reduce the rate of lung function decline and increase life expectancy [18]. However, antifibrotics may not be useful for the majority of people with PC19-PF. Antifibrotics may be effective in preventing progressive fibrosis in patients with established radiological evidence of fibrosis and a risk of advancement. Unfortunately, there is no proof for or against this method at this time. According to this research, all patients presenting with dyspnea in the chronic COVID-19 period should be evaluated for antifibrotic therapy. In patients whose dyspnea complaint did not regress, despite long-term steroid therapy, steroid-induced comorbidities and atrophy that arose, initially in the extremities and respiratory muscles, can lead to a vicious circle. Antifibrotic treatment that is started early in patients with fibrosis, despite antiinflammatory treatment, shows that positive results may be observed in > 5%, in both PFTs and quality of life of individuals [41]. To summarize, it is not known whether patients after COVID-19 who present with signs of pulmonary fibrosis should receive specific antifibrotic drugs, due to the fact that pulmonary fibrosis after COVID-19 is typically nonprogressive. Patients who do present with these signs should be monitored closely. Hence, antifibrotic medication may be recommended in the event that an underlying progressive interstitial lung disease (also known as IPF) has been luckily identified.
Following COVID-19 infection, lung involvement should be followed for up to 3 months, and it should be emphasized that there is a chance of regression. In other words, antifibrotic treatment is not required immediately. If fibrosis persists at the end of the 12th week, there is a case to be made for using these agents to treat PC19-PF, despite the lack of sufficient data. Pirfenidone or nintedanib should be used for at least 1–3 months to have an accurate measurement of the antifibrotic response [14].
Impact of Immunosuppression
Few studies have examined the effects of glucocorticoids and selective interleukin inhibition on PC19-PF, and those that have are conflicting. It is still possible to reduce the risk of pulmonary fibrosis by using immunomodulatory agents that reduce inflammation and the duration of mechanical ventilation.
A subgroup of patients with radiographic abnormalities consistent with PASC with organising pneumonia was described [39,40,41,42]. These patients had trouble getting off of mechanical support or still had low oxygen levels months after the initial infection [39]. It is not clear if COVID-19-related organizing pneumonia is linked to the development of pulmonary fibrosis or if corticosteroid treatment might lower the risk of PC19-PF, but these ideas should be carefully looked at in a future study [43,44,45].
Additionally, the role of prolonged treatment with corticosteroids in preventing PC19-PF is still uncertain. It seems that this treatment could be beneficial for a select group of patients in particular, such as tomographic anomalies suggesting organized pneumonia. In addition to clinical findings, antifibrotic response should be objectively assessed using high resolution CT (HRCT), diffusion lung capacity for carbon monoxide (DLCO), and 6-min walking test (MWT) [46]. In addition, Tanvir et al. found in an open label pilot research that encompassed 60 COVID-19 patients that 17 of the participants were given PFN and 19 of the participants were given corticosteroids. In the beginning of the trial, the parameters were analyzed, and at the end of the period, the antifibrotic effects of PFN were shown to be greater than those of corticosteroids. According to these observations, early therapy with PFN in severely affected individuals with COVID-19 may minimize the likelihood of developing post-COVID-19 pulmonary fibrosis [47]. So, concurrent use of steroids and antifibrotics is also being investigated, but the long-term outcomes are unknown.
Rehabilitation and Nonphamacological Management
In general, nonpharmacological therapy and rehabilitation help alleviate patients’ severe PC19-PF symptoms and enhance their quality of life. According to recent clinical guidelines, pulmonary rehabilitation, which includes exercise training, education, and behavioral changes, has the potential to improve physical and psychological conditions [48]. Patients with respiratory illnesses, and mixed respiratory and surgical populations can improve muscle strength, walking ability, and functional ability, with significant positive effects in the 6-min walking test (MWT) and Barthel index [49]. Pulmonary rehabilitation has been used successfully in severe cases of PF. Changes in behavior on the part of the patient may be necessary for successful pulmonary rehabilitation. These adjustments may involve losing weight, starting a regular routine for exercise, learning how to use breathing techniques, pacing, energy conservation strategies, and gaining an understanding of how to use medications, supplementary oxygen, and associated equipment. On the other hand, it has been demonstrated that oxygen therapy is beneficial in IPF. Some patients frequently experience hypoxemia, which can have an effect on their quality of life as it relates to their health. Its primary symptoms include lower levels of energy, as well as poorer social and physical functioning during the daytime. Patients who exhibit resting hypoxemia or severe oxygen desaturation during exercise are likely to see an improvement in their symptoms, as well as in their general quality of life, if they get supplementary oxygen therapy. As a consequence of this, oxygen therapy is an essential part of the treatment of pulmonary fibrosis, particularly PC10-PF [50].
Future Considerations
Pulmonary fibrosis can occur in a variety of clinical settings. Future studies are required to learn more about the pathogenesis, clinical manifestations, and psychosocial effects of this condition on patients. Clinical and serologic phenotyping of patients with PC19-PF, as well as comprehensive epidemiologic identification of particular risk variables, should be pursued as additional research directions. Longer follow-up studies are needed to determine how PC19-PF progresses and what the best long-term approach is for such patients, especially for those with ARDS, during the acute stage of COVID-19 infection [51, 52].
Conclusion
One of the most serious long-term consequences in COVID-19 patients is pulmonary fibrosis. The significant concerns concerning pulmonary fibrosis and the optimization of respiratory follow-up following COVID-19 are expected to be resolved in the near future. In addition, advanced age with limited lung function and preexisting comorbidities, such as diabetes, cardiovascular disease, hypertension, and obesity, increase the risk of developing fibrotic lung alterations in survivors with reduced exercise tolerance. Antifibrotic drugs are currently undergoing clinical trials. However, for improving the PC19-PF patients’ quality of life, the detailed follow-up and personalized rehabilitation should be encouraged.
References
Mohammadi A, Balan I, Yadav S, et al. Post-COVID-19 pulmonary fibrosis. Cureus. 2022;14(3):e22770.
Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–32.
Mylvaganam RJ, Bailey JI, Sznajder JI, et al. Recovering from a pandemic: pulmonary fibrosis after SARS-CoV-2 infection. Eur Respir Rev. 2021;30: 210194.
Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–15.
Lee JH, Yim JJ, Park J. Pulmonary function and chest computed tomography abnormalities 6–12 months after recovery from COVID-19: a systematic review and meta-analysis. Respir Res. 2022;23(1):233. https://doi.org/10.1186/s12931-022-02163-x. (Published 2022 Sep 6).
d’Ettorre G, Gentilini Cacciola E, Santinelli L, et al. Covid-19 sequelae in working age patients: a systematic review. J Med Virol. 2022;94(3):858–68. https://doi.org/10.1002/jmv.27399.
Mongelli A, Barbi V, Gottardi Zamperla M, et al. Evidence for Biological Age Acceleration and Telomere Shortening in COVID-19 Survivors. Int J Mol Sci. 2021;22(11):6151. https://doi.org/10.3390/ijms22116151. (Published 2021 Jun 7).
Rai DK, Sharma P, Kumar R. Post covid 19 pulmonary fibrosis. Is it real threat? Indian J Tuberc. 2021;68(3):330–3. https://doi.org/10.1016/j.ijtb.2020.11.003.
Groff D, Sun A, Ssentongo AE, Ba DM, Parsons N, Poudel GR, et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open. 2021;4: e2128568.
Wu C, Chen X, Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan. China JAMA Intern Med. 2020;180(7):934–43.
Liu X, Zhou H, Zhou Y. Risk factors associated with disease severity and length of hospital stay in COVID-19 patients. J Infect. 2020;81(1):e95–7.
Tanni SE, Fabro AT, de Albuquerque A, Machado Ferreira EV, Verrastro CGY, Sawamura MVY, et al. Pulmonary fibrosis secondary to COVID-19: a narrative review. Expet Rev Respir Med. 2021;15:791–803.
Oronsky B, Larson C, Hammond TC, Oronsky A, Kesari S, et al. A Review of Persistent Post-COVID Syndrome (PPCS). Clin Rev Allergy Immunol. 2021;20:1–9.
Esendağli D. Post-COVID syndrome: pulmonary complications. Turk J Med Sci. 2021;51:3359–71.
Bellan M, Soddu D, Balbo PE, et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. 2021;4: e2036142.
Abdallah SJ, Voduc N, Corrales-Medina VF, et al. Pulmonary function and functional capacity four months after COVID-19. Ann Am Thorac Soc. 2021;18:1912–7.
Vianello, et al. Pulmonary fibrosis in COVID-19 survivors. Clin Chem Lab Med. 2022;60(3):307–16.
Vasarmidi E, Tsitoura E, Spandidos DA, Tzanakis N, Antoniou KM. Pulmonary fibrosis in the aftermath of the COVID-19 era. Exp Ther Med. 2020;20:2557–60.
Burnham EL, Janssen WJ, Riches DWH, et al. The fibroproliferative response in acute respiratory distress syndrome: mechanisms and clinical significance. Eur Respir J. 2014;43:276–85.
Pelosi P, Rocco PR. Effects of mechanical ventilation on the extracellular matrix. Intensive Care Med. 2008;34:631–9.
Leng L, Cao R, Ma J, et al. Pathological features of COVID-19-associated lung injury: a preliminary proteomics report based on clinical samples. Signal Transduct Target Ther. 2020;5:240. https://doi.org/10.1038/s41392-020-00355-9.
Gentile F, Aimo A, Forfori F, et al. COVID-19 and risk of pulmonary fibrosis: the importance of planning ahead. Eur J Prev Cardiol. 2020;27:1442–6. https://doi.org/10.1177/2047487320932695.
John AE. COVID-19 and pulmonary fibrosis: a potential role for lung epithelial cells and fibroblasts. Immunol Rev. 2021;302:228–40.
George PM, Barratt SL, Condliffe R, et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax. 2020;75:1009–16.
Torres-Castro R, Vasconcello-Castillo L, Alsina-Restoy X, et al. Respiratory function in patients post-infection by COVID-19: a systematic review and meta-analysis. Pulmonology. 2021;27:328–37.
Solomon JJ, Heyman B, Ko JP, et al. CT of postacute lung complications of COVID-19. Radiology. 2021;301: 211396.
Wu X, Liu X, Zhou Y, Yu H, Li R, Zhan Q, et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021. https://doi.org/10.1016/S2213-2600(21)00174-0. (published online ahead of print, 2021 May 5).
Han X, Fan Y, Alwalid O, Li N, Jia X, Yuan M, et al. Six-month follow- up chest CT findings after severe COVID-19 pneumonia. Radiology. 2021;299(1):E177–86. https://doi.org/10.1148/radiol.2021203153.
Alarcón-Rodríguez J, Fernández-Velilla M, Ureña-Vacas A, et al. Radiological management and follow-up of post-COVID-19 patients. Radiologia. 2021;63:258.
Mandal S, Barnett J, Brill SE, et al. “Long-COVID”: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021;76:396–8.
McGroder CF, Zhang D, Choudhury MA, Salvatore MM, D’Souza BM, Hoffman EA, et al. Pulmonary fibrosis 4 months after COVID-19 is associated with severity of illness and blood leukocyte telomere length. Thorax. 2021. https://doi.org/10.1136/thoraxjnl-2021-217031. (published online ahead of print, 2021 Apr 29).
D’Cruz R, Waller M, Perrin F, et al. Chest radiography is a poor predictor of respiratory symptoms and functional impairment in survivors of severe COVID-19 pneumonia. ERJ Open Res. 2021;7:00655–2020.
Baldi BG, Tanni SE. Pulmonary fibrosis and follow-up of COVID-19 survivors: an urgent need for clarification. J Bras Pneumol. 2021;47(4): e20210213.
Arnold DT, Donald C, Lyon M, Hamilton FW, Morley AJ, Attwood M, et al. Krebs von den Lungen 6 (KL-6) as a marker for disease severity and persistent radiological abnormalities following COVID-19 infection at 12 weeks. PLoS One. 2021;16(4): e0249607. https://doi.org/10.1371/journal.pone.0249607.
Ko UW, Cho EJ, Oh HB, Koo HJ, Do KH, Song JW. Serum Krebs von den Lungen-6 level predicts disease progression in interstitial lung disease. PLoS One. 2020;15: e0244114. https://doi.org/10.1371/journal.pone.0244114.
Peng DH, Luo Y, Huang LJ, et al. Correlation of Krebs von den Lungen-6 and fibronectin with pulmonary fibrosis in coronavirus disease 2019. Clin Chim Acta. 2021;517:48–53. https://doi.org/10.1016/j.cca.2021.02.012.
Inoue Y, Kaner RJ, Guiot J, et al. Diagnostic and prognostic biomarkers for chronic fibrosing interstitial lung diseases wth a progressive phenotype. Chest. 2020;158:646–59. https://doi.org/10.1016/j.chest.2020.03.037.
George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020;8:807–15.
Al-kuraishy HM, Batiha GE-S, Faidah H, Al-Gareeb AI, Saad HM, Simal-Gandara J. Pirfenidone and post-Covid-19 pulmonary fibrosis: invoked again for realistic goals. Inflammopharmacology. 2022;30:2017–26.
Yoon HY, Park S, Kim DS, Song JW. Efficacy and safety of nintedanib in patients with advanced idiopathic pulmonary fibrosis. Respir Res. 2018;19:203.
Kerget B, CIL G, Araz O, Alper F, Akgun M. When and how important is anti-fibrotic therapy in the postCOVID-19 period? Bratisl Med J. 2022;123(9):653–8.
Vadász I, Husain-Syed F, Dorfmüller P, et al. Severe organising pneumonia following COVID-19. Thorax. 2021;76:201–4.
Kory P, Kanne JP. SARS-CoV-2 organising pneumonia: “Has there been a widespread failure to identify and treat this prevalent condition in COVID-19?” BMJ Open Respir Res. 2020;7: e000724.
Wijsenbeek M, Cottin V. Spectrum of fibrotic lung diseases. N Engl J Med. 2020;383:958–68.
Shah W, Hillman T, Playford ED, Hishmeh L. Managing the long term effects of covid-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ. 2021;372:n136. https://doi.org/10.1136/bmj.n136.
Chaudhary S, Natt B, Bime C, Knox KS, Glassberg MK. Antifibrotics in COVID-19 lung disease: let us stay focused. Front Med (Lausanne). 2020;7:539. https://doi.org/10.3389/fmed.2020.00539.
Tanvir M, Wagay I, Nisar S, Ahmed RN, Maqbool M, Kareem O, Muzaffer U. 2022 Early intervention with anti-fibrotic pirfenidone is effective than corticosteroids in preventing pulmonary fibrosis in severe COVID pneumonia patients. Curr Med Res Pract. 2022;12(2):53. https://doi.org/10.4103/cmrp.cmrp_110_21.
Reina-Gutiérrez S, Torres-Costoso A, Martínez-Vizcaíno V, de Arenas-Arroyo SN, Fernández-Rodríguez R, Pozuelo-Carrascosa DP. Effectiveness of pulmonary rehabilitation in interstitial lung disease, including coronavirus diseases: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2021;102:1989-97.e3. https://doi.org/10.1016/j.apmr.2021.03.035.
Goodwin VA, Allan L, Bethel A, et al. Rehabilitation to enable recovery from COVID-19: a rapid systematic review. Physiotherapy. 2021;111:4–22. https://doi.org/10.1016/j.physio.2021.01.007.
Egan JJ. Follow-up and nonpharmacological management of the idiopathic pulmonary fibrosis patient. Eur Respir Rev. 2011;20:114–7. https://doi.org/10.1183/09059180.00001811.
Duong-Quy S, Huynh-Truong-Anh D, Nguyen-Thi-Kim T, Nguyen-Quang T, Nguyen-Chi T, Nguyen-Thi-Y N, Duong-Thi-Thanh V, Ngo C, Craig T. The use of therapeutic plasma exchange in the treatment of a pregnant woman with COVID-19 induced acute respiratory distress syndrome. Pulmonary Therapy. 2022;8(2):233–40.
Duong-Quy S, Huynh-Truong-Anh D, Le-Thi-Hong N, Le-Van T, Le-Thi-Kim S, Nguyen-Quang T, Nguyen-Thi-Kim T, Nguyen-Phuong N, Nguyen-Chi T, Nguyen-Van T, Duong-Thi-Thanh V. Acute respiratory distress syndrome associated with multisystem inflammatory syndrome in a child with Covid-19 and diabetic ketoacidosis: a case report. Pulm Ther. 2022;24:1.
Acknowledgements
Funding
No funding or sponsorship was received for this review or publication charge.
Author Contributions
Conceptualization was carried out by S. Duong-Quy, T. Vo-Pham-Minh, V. Nguyen-Nhu; Methodology, S. Duong-Quy, T. Vo-Pham-Minh, Q. Tran-Xuan, T. Huynh-Anh, T. Vo-Van, Q. Vu-Tran-Thien, and V. Nguyen-Nhu. Validation was performed by S. Duong-Quy, T. Vo-Pham-Minh, and V. Nguyen-Nhu. Resources were collected by S. Duong-Quy and V. Nguyen-Nhu. Writing—original draft preparation—was performed by S. Duong-Quy, T. Vo-Pham-Minh, Q. Tran-Xuan, T. Huynh-Anh, T. Vo-Van, Q. Vu-Tran-Thien, and V. Nguyen-Nhu. Writing—review and editing—was performed by S. Duong-Quy, T. Vo-Pham-Minh, and V. Nguyen-Nhu. Project administration was carried out by S. Duong-Quy.
Disclosures
Sy Duong-Quy, Thu Vo-Pham-Minh, Quynh Tran-Xuan, Tuan Huynh-Anh, Tinh Vo-Van, Quan Vu-Tran-Thien, and Vinh Nguyen-Nhu have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
The data related to this Review are available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Duong-Quy, S., Vo-Pham-Minh, T., Tran-Xuan, Q. et al. Post-COVID-19 Pulmonary Fibrosis: Facts—Challenges and Futures: A Narrative Review. Pulm Ther 9, 295–307 (2023). https://doi.org/10.1007/s41030-023-00226-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41030-023-00226-y