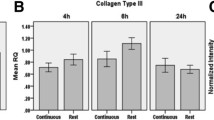
Abstract
Tendons and ligaments are collagenous connective tissues involved in locomotion and stabilization of joints. These tissues possess relatively low cellularity and vascularity, resulting in long and potentially incomplete healing responses following injury. For sub-failure injuries such as strains and sprains, the common treatment is an implementation of rest, ice, compression, and elevation. This procedure relies on the tissue’s natural healing ability, leaving the tissue prone to possible re-injury and failure. As a potential aid in the healing process, we investigated the effects of thermal stress on human tenocytes in vitro. This method exploits the activity of heat shock proteins, which assist in cellular proliferation and protein assembly. Heat shock at 40, 44, and 48 °C was applied to human hamstring tenocytes for 5–20 min. Studies were performed to determine metabolic activity, proliferation, protein secretion, and gene expression of the cells shortly after heating. A scratch wound healing assay was performed to monitor migration of cells as they recovered from heat shock. The data showed increased cellular activity following 15 and 20 min of thermal conditioning at 44 and 48 °C. Protein secretion and expression of collagens types I and III and TGF-β1 suggest that the heat shock response of tenocytes is similar to that of natural wound healing. The results revealed different responses for different temperatures and different durations of heat shock. The scratch assay revealed that heat might hasten recovery times following injury. Although additional studies that investigate additional heat shock proteins with different cell lines must be performed, these initial results suggest that heat shock may be a potential therapeutic tool that should be further investigated for the treatment of sub-failure tendon and ligament injuries. Heat shock presents a potential aid for the regeneration of damaged musculoskeletal tissues. In this preliminary study of human hamstring tenocytes in vitro, the application of thermal stress for a short duration caused rapid proliferation of cells after they were allowed to recover. Furthermore, parallels were observed between the in vitro heat shock response and the natural wound healing process of tendons and ligaments. This information provides potential for heat shock to assist in healing damage tendon and ligament tissue. Future works will need to explore the effects of heat shock on a wider range of tendon and ligament cells, as well as develop methods of applying thermal stress to tissue in vivo.







Similar content being viewed by others
References
Freeman JW. Tissue engineering options for ligament healing. Bone Tissue Regen Insights. 2009;2:13–23.
Attia M, Huet E, Gossard C, Menashi S, Tassoni MC, Martelly I. Early events of overused supraspinatus tendons involve matrix metalloproteinases and EMMPRIN/CD147 in the absence of inflammation. Am J Sports Med. 2013;41(4):908–17. https://doi.org/10.1177/0363546512473817.
Vunjak-Novakovic G, Altman G, Horan R, Kaplan DL. Tissue engineering of ligaments. Annu Rev Biomed Eng. 2004;6:131–56. https://doi.org/10.1146/annurev.bioeng.6.040803.140037.
Ekwueme EC, Shah JV, Mohiuddin M, Ghebes CA, Crispim JF, Saris DB, et al. Cross-talk between human tenocytes and bone marrow stromal cells potentiates extracellular matrix remodeling in vitro. J Cell Biochem. 2016;117(3):684–93. https://doi.org/10.1002/jcb.25353.
Domnick C, Raschke MJ, Herbort M. Biomechanics of the anterior cruciate ligament: physiology, rupture and reconstruction techniques. World J Orthop. 2016;7(2):82–93. https://doi.org/10.5312/wjo.v7.i2.82.
Ekwueme EC, Kwansa AL, Sharif K, El-Amin SF, Freeman JW. Recent advancements in ligament replacement. Recent Pat Biomed Eng. 2011;4(3):196–204. https://doi.org/10.2174/1874764711104030196.
Hast MW, Zuskov A, Soslowsky LJ. The role of animal models in tendon research. Bone Joint Res. 2014;3(6):193–202. https://doi.org/10.1302/2046-3758.36.2000281.
Rothrauff BB, Tuan RS. Cellular therapy in bone-tendon interface regeneration. Organ. 2014;10(1):13–28. https://doi.org/10.4161/org.27404.
Kwan KH, Yeung KW, Liu X, Wong KK, Shum HC, Lam YW, et al. Silver nanoparticles alter proteoglycan expression in the promotion of tendon repair. Nanomedicine. 2014;10(7):1375–83. https://doi.org/10.1016/j.nano.2013.11.015.
Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87(1):187–202. https://doi.org/10.2106/JBJS.D.01850.
James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008;33(1):102–12. https://doi.org/10.1016/j.jhsa.2007.09.007.
Lynch SA, Renstrom PA. Treatment of acute lateral ankle ligament rupture in the athlete. Conservative versus surgical treatment Sports Med. 1999;27(1):61–71.
Forsyth AL, Zourikian N, Valentino LA, Rivard GE. The effect of cooling on coagulation and haemostasis: should “ice” be part of treatment of acute haemarthrosis in haemophilia? Haemophilia. 2012;18(6):843–50. https://doi.org/10.1111/j.1365-2516.2012.02918.x.
Kuo CK, Marturano JE, Tuan RS. Novel strategies in tendon and ligament tissue engineering: advanced biomaterials and regeneration motifs. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:20. https://doi.org/10.1186/1758-2555-2-20.
Kwansa AL, Empson YM, Ekwueme EC, Walters VI, Freeman JW, Laurencin CT. Novel matrix based anterior cruciate ligament (ACL) regeneration. Soft Matter. 2010;6(20):5016. https://doi.org/10.1039/c0sm00182a.
Barber JG, Handorf AM, Allee TJ, Li WJ. Braided nanofibrous scaffold for tendon and ligament tissue engineering. Tissue Eng Part A. 2013;19(11–12):1265–74. https://doi.org/10.1089/ten.tea.2010.0538.
Cooper JA Jr, Sahota JS, Gorum WJ 2nd, Carter J, Doty SB, Laurencin CT. Biomimetic tissue-engineered anterior cruciate ligament replacement. Proc Natl Acad Sci U S A. 2007;104(9):3049–54. https://doi.org/10.1073/pnas.0608837104.
Erisken C, Zhang X, Moffat KL, Levine WN, Lu HH. Scaffold fiber diameter regulates human tendon fibroblast growth and differentiation. Tissue Eng Part A. 2013;19(3–4):519–28. https://doi.org/10.1089/ten.tea.2012.0072.
Kashiwagi K, Mochizuki Y, Yasunaga Y, Ishida O, Deie M, Ochi M. Effects of transforming growth factor-beta 1 on the early stages of healing of the Achilles tendon in a rat model. Scand J Plast Reconstr Surg Hand Surg. 2004;38(4):193–7. https://doi.org/10.1080/02844310410029110.
Dahlgren LA, Mohammed HO, Nixon AJ. Expression of insulin-like growth factor binding proteins in healing tendon lesions. J Orthop Res. 2006;24(2):183–92. https://doi.org/10.1002/jor.20000.
Molloy T, Wang Y, Murrell GAC. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33(5):381–94.
Boyer MI, Watson JT, Lou J, Manske PR, Gelberman RH, Cai SR. Quantitative variation in vascular endothelial growth factor mRNA expression during early flexor tendon healing: an investigation in a canine model. J Orthop Res. 2001;19(5):869–72. https://doi.org/10.1016/S0736-0266(01)00017-1.
Watanabe N, Woo SL, Papageorgiou C, Celechovsky C, Takai S. Fate of donor bone marrow cells in medial collateral ligament after simulated autologous transplantation. Microsc Res Tech. 2002;58(1):39–44. https://doi.org/10.1002/jemt.10115.
Chan BP, S-c F, Qin L, K-m L, Rolf CG, K-m C. Effects of basic fibroblast growth factor (bFGF) on early stages of tendon healing: a rat patellar tendon model. Acta Orthop Scand. 2000;71(5):513–8.
Shibaguchi T, Sugiura T, Fujitsu T, Nomura T, Yoshihara T, Naito H, et al. Effects of icing or heat stress on the induction of fibrosis and/or regeneration of injured rat soleus muscle. J Physiol Sci. 2016;66(4):345–57. https://doi.org/10.1007/s12576-015-0433-0.
Hatade T, Takeuchi K, Fujita N, Arakawa T, Miki A. Effect of heat stress soon after muscle injury on the expression of MyoD and myogenin during regeneration process. J Musculoskel Neuron Interact. 2014;14(3):325–33.
Riederer I, Negroni E, Bigot A, Bencze M, Di Santo J, Aamiri A, et al. Heat shock treatment increases engraftment of transplanted human myoblasts into immunodeficient mice. Transplant Proc. 2008;40(2):624–30. https://doi.org/10.1016/j.transproceed.2008.01.026.
Harder Y, Contaldo C, Klenk J, Banic A, Jakob SM, Erni D. Improved skin flap survival after local heat preconditioning in pigs. J Surg Res. 2004;119(1):100–5. https://doi.org/10.1016/j.jss.2003.11.002.
Rylander MN, Diller KR, Wang S, Aggarwal SJ. Correlation of HSP70 expression and cell viability following thermal stimulation of bovine aortic endothelial cells. J Biomech Eng. 2005;127(5):751–7. https://doi.org/10.1115/1.1993661.
Lee MW, Muramatsu T, Uekusa T, Lee JH, Shimono M. Heat stress induces alkaline phosphatase activity and heat shock protein 25 expression in cultured pulp cells. Int Endod J. 2008;41(2):158–62. https://doi.org/10.1111/j.1365-2591.2007.01331.x.
Chung E, Sampson AC, Rylander MN. Influence of heating and cyclic tension on the induction of heat shock proteins and bone-related proteins by MC3T3-E1 cells. Biomed Res Int 2014;2014:354260. doi:https://doi.org/10.1155/2014/354260, 1, 16.
Shui C, Scutt A. Mild heat shock induces proliferation, alkaline phosphatase activity, and mineralization in human bone marrow stromal cells and Mg-63 cells in vitro. J Bone Miner Res. 2001;16(4):731–41. https://doi.org/10.1359/jbmr.2001.16.4.731.
Yoshida K, Uoshima K, Oda K, Maeda T. Influence of heat stress to matrix on bone formation. Clin Oral Implants Res. 2009;20(8):782–90. https://doi.org/10.1111/j.1600-0501.2008.01654.x.
Oberringer M, Baum HP, Jung V, Welter C, Frank J, Kuhlmann M, et al. Differential expression of heat shock protein 70 in well healing and chronic human wound tissue. Biochem Biophys Res Commun. 1995;214(3):1009–14. https://doi.org/10.1006/bbrc.1995.2386.
Mosser DD, Caron AW, Bourget L, Denis-Larose C, Massie B. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol Cell Biol. 1997;17(9):5317–27.
Helmbrecht K, Zeise E, Rensing L. Chaperones in cell cycle regulation and mitogenic signal transduction: a review. Cell Prolif. 2000;33:341–65.
Sherman MY, Gabai VL. Hsp70 in cancer: back to the future. Oncogene. 2015;34(32):4153–61. https://doi.org/10.1038/onc.2014.349.
Suzuki K, Watanabe M. Modulation of cell growth and mutation induction by introduction of the expression vector of human hsp70 gene. Exp Cell Res. 1994;215(1):75–81. https://doi.org/10.1006/excr.1994.1317.
Zeise E, Kuhl N, Kunz J, Rensing L. Nuclear translocation of stress protein Hsc70 during S phase in rat C6 glioma cells. Cell Stress Chaperones. 1998;3(2):94–9.
Yoshidomi K, Murakami A, Yakabe K, Sueoka K, Nawata S, Sugino N. Heat shock protein 70 is involved in malignant behaviors and chemosensitivities to cisplatin in cervical squamous cell carcinoma cells. J Obstet Gynaecol Res. 2014;40(5):1188–96. https://doi.org/10.1111/jog.12325.
Zhe Y, Li Y, Liu D, Su DM, Liu JG, Li HY. Extracellular HSP70-peptide complexes promote the proliferation of hepatocellular carcinoma cells via TLR2/4/JNK1/2MAPK pathway. Tumour Biol. 2016;37(10):13951–9. https://doi.org/10.1007/s13277-016-5189-5.
Wu J, Liu T, Rios Z, Mei Q, Lin X, Cao S. Heat shock proteins and cancer. Trends Pharmacol Sci. 2017;38(3):226–56. https://doi.org/10.1016/j.tips.2016.11.009.
Mala JG, Rose C. Interactions of heat shock protein 47 with collagen and the stress response: an unconventional chaperone model? Life Sci. 2010;87(19–22):579–86. https://doi.org/10.1016/j.lfs.2010.09.024.
Ito S, Nagata K. Biology of Hsp47 (serpin H1), a collagen-specific molecular chaperone. Semin Cell Dev Biol. 2017;62:142–51. https://doi.org/10.1016/j.semcdb.2016.11.005.
Ishida Y, Kubota H, Yamamoto A, Kitamura A, Bachinger HP, Nagata K. Type I collagen in Hsp47-null cells is aggregated in endoplasmic reticulum and deficient in N-propeptide processing and fibrillogenesis. Mol Biol Cell. 2006;17(5):2346–55. https://doi.org/10.1091/mbc.E05-11-1065.
Satoh M, Hirayoshi K, Yokota S, Hosokawa N, Nagata K. Intracellular interaction of collagen-specific stress protein HSP47 with newly synthesized procollagen. J Cell Biol. 1996;133(2):469–83.
Liang C-C, Park AY, Guan J-L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protocols. 2007;2(2):329–33.
Chung E, Rylander MN. Response of preosteoblasts to thermal stress conditioning and osteoinductive growth factors. Cell Stress Chaperones. 2012;17(2):203–14. https://doi.org/10.1007/s12192-011-0300-8.
Birch HL, Wilson AM, Goodship AE. The effect of exercise-induced localised hyperthermia on tendon cell survival. J Exp Biol. 1997;200(Pt 11):1703–8.
Hosaka Y, Ozoe S, Kirisawa R, Ueda H, Takehana K, Yamaguchi M. Effect of heat on synthesis of gelatinases and pro-inflammatory cytokines in equine tendinocytes. Biomed Res. 2006;27(5):233–41.
Hsu SL, Liang R, Woo SL. Functional tissue engineering of ligament healing. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:12. https://doi.org/10.1186/1758-2555-2-12.
Millar NL, Murrell GA. Heat shock proteins in tendinopathy: novel molecular regulators. Mediat Inflamm 2012;2012:436203. doi:https://doi.org/10.1155/2012/436203, 1, 7.
Milarski KL, Welch WJ, Morimoto RI. Cell cycle-dependent association of HSP70 with specific cellular proteins. J Cell Biol. 1989;108(2):413–23.
Wang R, Kovalchin JT, Muhlenkamp P, Chandawarkar RY. Exogenous heat shock protein 70 binds macrophage lipid raft microdomain and stimulates phagocytosis, processing, and MCH-II presentation of antigens. Blood. 2006;107(4):1636–42. https://doi.org/10.1182/blood-200506-2559.
Guzhova I, Kislyakova K, Moskaliova O, Fridlanskaya I, Tytell M, Cheetham M, et al. In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res. 2001;914:66–73.
Nagai N, Hosokawa M, Itohara S, Adachi E, Matsushita T, Hosokawa N, et al. Embryonic lethality of molecular chaperone Hsp47 knockout mice is associated with defects in collagen biosynthesis. J Cell Biol. 2000;150(6):1499–505.
Halper J, Griffin A, Hu W, Jung C, Zhang J, Pan H, et al. In vitro culture decreases the expression of TGF(beta), Hsp47 and type I procollagen and increases the expression of CTGF in avian tendon explants. J Musculoskelet Neuronal Interact. 2005;5(1):53–63.
Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18(7):816–27. https://doi.org/10.1096/fj.03-1273rev.
Li L, Klim JR, Derda R, Courtney AH, Kiessling LL. Spatial control of cell fate using synthetic surfaces to potentiate TGF-beta signaling. Proc Natl Acad Sci U S A. 2011;108(29):11745–50. https://doi.org/10.1073/pnas.1101454108.
Faulknor RA, Olekson MA, Nativ NI, Ghodbane M, Gray AJ, Berthiaume F. Mesenchymal stromal cells reverse hypoxia-mediated suppression of alpha-smooth muscle actin expression in human dermal fibroblasts. Biochem Biophys Res Commun. 2015;458(1):8–13. https://doi.org/10.1016/j.bbrc.2015.01.013.
Henninger HB, Valdez WR, Scott SA, Weiss JA. Elastin governs the mechanical response of medial collateral ligament under shear and transverse tensile loading. Acta Biomater. 2015;25:304–12. https://doi.org/10.1016/j.actbio.2015.07.011.
Fang F, Lake SP. Multiscale mechanical integrity of human supraspinatus tendon in shear after elastin depletion. J Mech Behav Biomed Mater. 2016;63:443–55. https://doi.org/10.1016/j.jmbbm.2016.06.032.
Smith KD, Clegg PD, Innes JF, Comerford EJ. Elastin content is high in the canine cruciate ligament and is associated with degeneration. Vet J. 2014;199(1):169–74. https://doi.org/10.1016/j.tvjl.2013.11.002.
Zhang J, Wang JH. The effects of mechanical loading on tendons—an in vivo and in vitro model study. PLoS One. 2013;8(8):e71740. https://doi.org/10.1371/journal.pone.0071740.
Lu H, Zheng C, Wang Z, Chen C, Chen H, Hu J. Effects of low-intensity pulsed ultrasound on new trabecular bone during bone-tendon junction healing in a rabbit model: a synchrotron radiation micro-CT study. PLoS One. 2015;10(4):e0124724. https://doi.org/10.1371/journal.pone.0124724.
Hu B, Zhang Y, Zhou J, Li J, Deng F, Wang Z, et al. Low-intensity pulsed ultrasound stimulation facilitates osteogenic differentiation of human periodontal ligament cells. PLoS One. 2014;9(4):e95168. https://doi.org/10.1371/journal.pone.0095168.
Yang Z, Ren L, Deng F, Wang Z, Song J. Low-intensity pulsed ultrasound induces osteogenic differentiation of human periodontal ligament cells through activation of bone morphogenetic protein-smad signaling. J Ultrasound Med. 2014;33(5):865–73. https://doi.org/10.7863/ultra.33.5.865.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors alone are responsible for the content and writing of the paper.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shah, J.V., Ekwueme, E.C. & Freeman, J.W. Investigation of the Short-term Effects of Heat Shock on Human Hamstring Tenocytes In Vitro. Regen. Eng. Transl. Med. 6, 50–61 (2020). https://doi.org/10.1007/s40883-018-0070-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-018-0070-2