Abstract
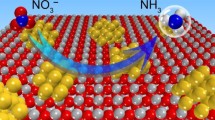
Electrochemical nitrate reduction to ammonia (NRA) is considered a promising strategy for environmental protection and energy saving because it can simultaneously achieve nitrate wastewater treatment and ammonia synthesis. However, currently, this method suffers from the lack of efficient electrocatalysts. In this work, through a “killing two birds with one stone” strategy, a catalyst, TiO2@C/Fe2O3 nanosheet arrays (NSAs), was fabricated by introducing hydrothermal carbon (HTC), which not only accelerates the charge transfer due to its good conductivity but also provides abundant oxygen vacancies owing to its nice reducibility, finally improving the NRA performance. Compared with TiO2/Fe2O3 NSAs, TiO2@C/Fe2O3 exhibited higher NRA performance with a nitrate conversion of 88.6%, ammonia selectivity of 83.4%, Faradaic efficiency of 85.3%, and ammonia yield of 0.2176 mmol h−1 cm−2. Moreover, the retention of high activity in nine cyclic experiments confirmed the outstanding stability of TiO2@C/Fe2O3. Furthermore, the possible NRA reaction pathway was derived from the results of electrochemical quasi in situ electron spin resonance experiments, electrochemical in situ attenuated total reflection Fourier transform infrared measurement and online differential electrochemical mass spectrometry. This feasible strategy of introducing an HTC interlayer may open new avenues for developing novel NRA electrocatalysts.

摘要
电化学硝酸根还原制氨(NRA)可以同时实现硝酸盐废水处理和氨合成, 是一种很有前途的环保节能策略. 目前构建高效的电催化剂是人们关注的焦点. 本文构建了TiO2@C/Fe2O3纳米片阵列作为高效催化剂, 采用多种表征手段系统研究了水热碳的引入对NRA性能的影响. 我们发现水热碳既可作为良导体实现电荷的快速传递, 又可作为还原剂引入更多的氧空位, 实现了“一石二鸟”催化剂构建策略. 与TiO2/Fe2O3相比, TiO2@C/Fe2O3具有更高的NRA性能, 硝酸盐转化率为88.6%、 氨选择性为8 3. 4 % 、 法拉第效率为8 5. 3 % 、氨收率为0.2176 mmol h−1cm−2. 此外, 在9次循环实验后, TiO2@C/Fe2O3的高活性仍得以保持, 证实了TiO2@C/Fe2O3出色的稳定性. 此外, 我们利用电化学准原位电子自旋共振实验、 电化学原位衰减全反射傅立叶变换红外测量和在线差分电化学质谱法推导了可能的NRA反应途径. 这种引入水热碳中间层的策略可为开发新型NRA电催化剂开辟新的途径.
Similar content being viewed by others
References
Wang Y, Zhou W, Jia R, et al. Unveiling the activity origin of a copper-based electrocatalyst for selective nitrate reduction to ammonia. Angew Chem Int Ed, 2020, 59: 5350–5354
Wu ZY, Karamad M, Yong X, et al. Electrochemical ammonia synthesis via nitrate reduction on Fe single atom catalyst. Nat Commun, 2021, 12: 2870
Ashida Y, Arashiba K, Nakajima K, et al. Molybdenum-catalysed ammonia production with samarium diiodide and alcohols or water. Nature, 2019, 568: 536–540
Tang C, Qiao SZ. How to explore ambient electrocatalytic nitrogen reduction reliably and insightfully. Chem Soc Rev, 2019, 48: 3166–3180
Wan Y, Xu J, Lv R. Heterogeneous electrocatalysts design for nitrogen reduction reaction under ambient conditions. Mater Today, 2019, 27: 69–90
Kyriakou V, Garagounis I, Vourros A, et al. An electrochemical Haber-Bosch process. Joule, 2020, 4: 142–158
Suryanto BHR, Du HL, Wang D, et al. Challenges and prospects in the catalysis of electroreduction of nitrogen to ammonia. Nat Catal, 2019, 2: 290–296
Wang H, Mao Q, Ren T, et al. Synergism of interfaces and defects: Cu/oxygen vacancy-rich Cu-Mn3O4 heterostructured ultrathin nanosheet arrays for selective nitrate electroreduction to ammonia. ACS Appl Mater Interfaces, 2021, 13: 44733–44741
Qing G, Ghazfar R, Jackowski ST, et al. Recent advances and challenges of electrocatalytic N2 reduction to ammonia. Chem Rev, 2020, 120: 5437–5516
Andersen SZ, Čolić V, Yang S, et al. A rigorous electrochemical ammonia synthesis protocol with quantitative isotope measurements. Nature, 2019, 570: 504–508
He W, Zhang J, Dieckhöfer S, et al. Splicing the active phases of copper/cobalt-based catalysts achieves high-rate tandem electroreduction of nitrate to ammonia. Nat Commun, 2022, 13: 1129
Nolan BT, Hitt KJ, Ruddy BC. Probability of nitrate contamination of recently recharged groundwaters in the conterminous United States. Environ Sci Technol, 2002, 36: 2138–2145
Butcher Jr DP, Gewirth AA. Nitrate reduction pathways on Cu single crystal surfaces: Effect of oxide and Cl−. Nano Energy, 2016, 29: 457–465
Rezvani F, Sarrafzadeh MH, Ebrahimi S, et al. Nitrate removal from drinking water with a focus on biological methods: A review. Environ Sci Pollut Res, 2019, 26: 1124–1141
Labarca F, Bórquez R. Comparative study of nanofiltration and ion exchange for nitrate reduction in the presence of chloride and iron in groundwater. Sci Total Environ, 2020, 723: 137809
van Langevelde PH, Katsounaros I, Koper MTM. Electrocatalytic nitrate reduction for sustainable ammonia production. Joule, 2021, 5: 290–294
Martínez J, Ortiz A, Ortiz I. State-of-the-art and perspectives of the catalytic and electrocatalytic reduction of aqueous nitrates. Appl Catal B-Environ, 2017, 207: 42–59
Bae BU, Jung YH, Han WW, et al. Improved brine recycling during nitrate removal using ion exchange. Water Res, 2002, 36: 3330–3340
Wang Y, Wang C, Li M, et al. Nitrate electroreduction: Mechanism insight, in situ characterization, performance evaluation, and challenges. Chem Soc Rev, 2021, 50: 6720–6733
Ren T, Ren K, Wang M, et al. Concave-convex surface oxide layers over copper nanowires boost electrochemical nitrate-to-ammonia conversion. Chem Eng J, 2021, 426: 130759
Jia R, Wang Y, Wang C, et al. Boosting selective nitrate electroreduction to ammonium by constructing oxygen vacancies in TiO2. ACS Catal, 2020, 10: 3533–3540
Zhang X, Wang C, Guo Y, et al. Cu clusters/TiO2−x with abundant oxygen vacancies for enhanced electrocatalytic nitrate reduction to ammonia. J Mater Chem A, 2022, 10: 6448–6453
Kong D, Wang H, Lu Z, et al. CoSe2 nanoparticles grown on carbon fiber paper: An efficient and stable electrocatalyst for hydrogen evolution reaction. J Am Chem Soc, 2014, 136: 4897–4900
Ge X, Chen L, Zhang L, et al. Nanoporous metal enhanced catalytic activities of amorphous molybdenum sulfide for high-efficiency hydrogen production. Adv Mater, 2014, 26: 3100–3104
Wu X, Ma A, Hu J, et al. Amorphous nickel-iron hydroxide nanosheets for effective electroreduction of nitrate to ammonia. Inorg Chem Front, 2023, 10: 666–674
Zhang Z, Liu C, Liu D, et al. Hydrothermal carbon-supported Ni catalysts for selective hydrogenation of 5-hydroxymethylfurfural toward tunable products. J Mater Sci, 2020, 55: 14179–14196
Liao JY, Higgins D, Lui G, et al. Multifunctional TiO2-C/MnO2 core-double-shell nanowire arrays as high-performance 3D electrodes for lithium ion batteries. Nano Lett, 2013, 13: 5467–5473
Liu C, He Y, Wei L, et al. Hydrothermal carbon-coated TiO2 as support for Co-based catalyst in Fischer–Tropsch synthesis. ACS Catal, 2018, 8: 1591–1600
Zou Z, Yang X, Zhang P, et al. Trace carbon-hybridized ZnS/ZnO hollow nanospheres with multi-enhanced visible-light photocatalytic performance. J Alloys Compd, 2019, 775: 481–489
Guo Y, Zhang R, Zhang S, et al. Pd doping-weakened intermediate adsorption to promote electrocatalytic nitrate reduction on TiO2 nanoarrays for ammonia production and energy supply with zinc-nitrate batteries. Energy Environ Sci, 2021, 14: 3938–3944
Zhang X, Wang Y, Liu C, et al. Recent advances in non-noble metal electrocatalysts for nitrate reduction. Chem Eng J, 2021, 403: 126269
Chen X, Liu L, Yu PY, et al. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science, 2011, 331: 746–750
Sun X, Yan X, Su H, et al. Non-stacked γ-Fe2O3/C@TiO2 double-layer hollow nanoparticles for enhanced photocatalytic applications under visible light. Nanomaterials, 2022, 12: 201
Shi J, Chen G, Zeng G, et al. Hydrothermal synthesis of graphene wrapped Fe-doped TiO2 nanospheres with high photocatalysis performance. Ceramics Int, 2018, 44: 7473–7480
Kontos AI, Likodimos V, Stergiopoulos T, et al. Self-organized anodic TiO2 nanotube arrays functionalized by iron oxide nanoparticles. Chem Mater, 2009, 21: 662–672
Fujii T, de Groot FMF, Sawatzky GA, et al. In situ XPS analysis of various iron oxide films grown by NO2-assisted molecular-beam epitaxy. Phys Rev B, 1999, 59: 3195–3202
Cao C, Li X, Zha Y, et al. Crossed ferric oxide nanosheets supported cobalt oxide on 3-dimensional macroporous Ni foam substrate used for diesel soot elimination under self-capture contact mode. Nanoscale, 2016, 8: 5857–5864
Gong Z, Zhong W, He Z, et al. Regulating surface oxygen species on copper(I) oxides via plasma treatment for effective reduction of nitrate to ammonia. Appl Catal B-Environ, 2022, 305: 121021
Bao J, Zhang X, Fan B, et al. Ultrathin spinel-structured nanosheets rich in oxygen deficiencies for enhanced electrocatalytic water oxidation. Angew Chem Int Ed, 2015, 54: 7399–7404
Han S, Li H, Li T, et al. Ultralow overpotential nitrate reduction to ammonia via a three-step relay mechanism. Nat Catal, 2023, 6: 402–414
Li T, Han S, Cheng C, et al. Sulfate-enabled nitrate synthesis from nitrogen electrooxidation on a rhodium electrocatalyst. Angew Chem, 2022, 134: e202204541
Yao Y, Zhu S, Wang H, et al. A spectroscopic study on the nitrogen electrochemical reduction reaction on gold and platinum surfaces. J Am Chem Soc, 2018, 140: 1496–1501
Wang Y, Li H, Zhou W, et al. Structurally disordered RuO2 nanosheets with rich oxygen vacancies for enhanced nitrate electroreduction to ammonia. Angew Chem Int Ed, 2022, 61: e202202604
Pérez-Gallent E, Figueiredo MC, Katsounaros I, et al. Electrocatalytic reduction of nitrate on copper single crystals in acidic and alkaline solutions. Electrochim Acta, 2017, 227: 77–84
Fang JY, Zheng QZ, Lou YY, et al. Ampere-level current density ammonia electrochemical synthesis using CuCo nanosheets simulating nitrite reductase bifunctional nature. Nat Commun, 2022, 13: 7899
Liu JX, Richards D, Singh N, et al. Activity and selectivity trends in electrocatalytic nitrate reduction on transition metals. ACS Catal, 2019, 9: 7052–7064
Gao J, Jiang B, Ni C, et al. Enhanced reduction of nitrate by noble metal-free electrocatalysis on P doped three-dimensional Co3O4 cathode: Mechanism exploration from both experimental and DFT studies. Chem Eng J, 2020, 382: 123034
Chaplin BP, Reinhard M, Schneider WF, et al. Critical review of Pd-based catalytic treatment of priority contaminants in water. Environ Sci Technol, 2012, 46: 3655–3670
Deng X, Yang Y, Wang L, et al. Metallic Co nanoarray catalyzes selective NH3 production from electrochemical nitrate reduction at current densities exceeding 2 A cm−2. Adv Sci, 2021, 8: 2004523
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21576211), Tianjin Science and Technology Program (22ZYJDSS00060 and 22YDTPJC00920), the Program for Tianjin Innovative Research Team in Universities (TD13-5031), and Tianjin 131 Research Team of Innovative Talents.
Author information
Authors and Affiliations
Contributions
Author contributions Wu X performed the experiments, analyzed the data and wrote the draft with support from Ma A, Liu D, Li X, Zhou Y and Mamba BB. Kuvarega AT revised the paper. Li H and Gui J supervised the project. All authors contributed to the general discussion.
Corresponding authors
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Supplementary information Experimental details and supporting data are available in the online version of the paper.
Xuan Wu is a doctoral candidate at the School of Material Science and Technology, Tiangong University. She graduated from Tiangong University with a Bachelor’s degree in 2018. Her research is focused on the design and synthesis of advanced nanomaterials for electrocatalytic nitrate reduction.
Aijing Ma is an associate professor at the School of Chemical Engineering and Technology, Tiangong University. She received her PhD degree in 2015 from the University of South Australia. She completed her postdoctoral fellowship from Nanyang Technological University, Singapore in 2016 and then joined Tiangong University. Her current research interests mainly focus on the design and synthesis of advanced materials for energy and environmental catalysis.
Dan Liu received her PhD degree from China University of Petroleum. After postdoctoral training at Korea Research Institute of Chemical Technology for three years and a one-year academic visit at Brown University, she is currently a professor at Tiangong University and was awarded Tianjin Distinguished Professor in 2020. Her main research interest focuses on environmental catalysis, low-carbon technology and smart nanomaterials.
Hu Li is a president & senior engineer at the Institute of Coal Chemical Industry Technology, Ningxia Coal Industry Co., Ltd. affiliated to China Energy Group. His research fields range from the coal-to-oil process to diversified indirect coal liquefaction downstream products, e.g., long-chain alcohol and lubricating base oil. He won the Science and Technology Progress Awards from China Petroleum and Chemical Federation (Grand Prize, 2018), CHN Energy (Grand Prize, 2019), and Ningxia Hui Autonomous Region (second prize, 2020).
Jianzhou Gui received his PhD degree from Lanzhou University in 2005, and became a senior visiting scholar at Korea Research Institute of Chemical Technology in 2009. He is currently the dean of Tianjin Key Laboratory of Green Chemical Technology and Process Engineering, and a professor of the School of Chemical Engineering and Technology, Tiangong University. His research interests include petrochemical engineering, green catalysis, adsorption and separation, and functional materials.
Rights and permissions
About this article
Cite this article
Wu, X., Ma, A., Liu, D. et al. Nitrate electroreduction to ammonia over TiO2@C/Fe2O3 nanosheet arrays: Unraveling the impact of hydrothermal carbon. Sci. China Mater. 66, 4367–4376 (2023). https://doi.org/10.1007/s40843-023-2620-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-023-2620-4