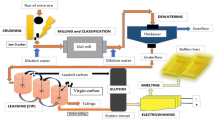
Abstract
Yulong copper mine is located in the Changdu region in the eastern mountainous area of Tibetan Plateau, where the elevation of mining area is over 4500 m. At present, the oxide ores from Yulong No. II bodies are refractory to direct agitation leaching in sulfuric acid media under normal leaching conditions. In this research, the mineralogical characteristics of this type of copper ores have been examined using automated mineral liberation analysis (MLA) integrated with scanning electron microscopy. The results showed that this type of copper ores had an extremely high binding rate due to the encapsulation of copper-bearing minerals within gangue minerals, such as kaolinite clay. For the agitation leaching process of Yulong copper ores in sulfuric acid media, the effects of leaching parameters were investigated on copper extraction, aluminum and iron dissolution, respectively. The studied parameters include liquid/solid ratio, agitation speed, leaching temperature, initial sulfuric acid concentration and particle size. The results of kinetic analysis of the leaching data under various leaching temperature indicated that the agitation leaching of copper ores belongs to diffusion control, giving an activation energy of 6.86 kJ/mol for copper extraction, 6.99 kJ/mol for aluminum dissolution and 14.96 kJ/mol for iron dissolution, respectively. Copper recovery from agitation leaching after ball milling as high as 85% was obtained, whereas the direct leaching process resulted in the recovery of only 60% in sulfuric acid solutions. The high-efficiency and low-cost recovery method for this type of oxide copper ore from Tibet Yulong Mines still needs further development in the future.
Graphical Abstract
















Similar content being viewed by others
References
Domarev V (1962) Basic features of the metallogeny of copper. Int Geol Rev 4(3):263–270. https://doi.org/10.1080/00206816209473685
Ji G, Liao Y, Wu Y, Xi J, Liu Q (2022) A review on the research of hydrometallurgical leaching of low-grade complex chalcopyrite. J Sustain Metall 8(3):964–977. https://doi.org/10.1007/s40831-022-00561-5
Schlesinger ME, Sole KC, Davenport WG, Alvear GR (2021) Extractive metallurgy of copper. Elsevier
Asghari M, Nakhaei F, VandGhorbany O (2019) Copper recovery improvement in an industrial flotation circuit: a case study of Sarcheshmeh copper mine. Energ Source Part A 41(6):761–778. https://doi.org/10.1080/15567036.2018.1520356
Xi J, Liao Y, Ji G, Liu Q, Wu Y (2022) Mineralogical characteristics and oxygen pressure acid leaching of low-grade polymetallic complex chalcopyrite. J Sustain Metall 8(4):1628–1638. https://doi.org/10.1007/s40831-022-00594-w
Norgate T, Jahanshahi S (2010) Low grade ores-smelt, leach or concentrate? Miner Eng 23(2):65–73. https://doi.org/10.1016/j.mineng.2009.10.002
Hao X, Liang Y, Yin H, Ma L, Xiao Y, Liu Y, Qiu G, Liu X (2016) The effect of potential heap construction methods on column bioleaching of copper flotation tailings containing high levels of fines by mixed cultures. Miner Eng 98:279–285. https://doi.org/10.1016/j.mineng.2016.07.015
Jallad K, Ben-Amotz D (2001) Chemical imaging of iron oxides and oxyhydroxides using near infrared Raman imaging microscopy. Mater Sci Tech-lond 17(11):1479–1486. https://doi.org/10.1179/026708301101509502
Rossel RV, McGlynn R, McBratney A (2006) Determining the composition of mineral-organic mixes using UV-vis-NIR diffuse reflectance spectroscopy. Geoderma 137(1–2):70–82. https://doi.org/10.1016/j.geoderma.2006.07.004
Nicol MJ, Akilan C (2018) The kinetics of the dissolution of chrysocolla in acid solutions. Hydrometallurgy 178:7–11. https://doi.org/10.1016/j.hydromet.2018.04.001
Chou E, Queneau P, Rickard R (1977) Sulfuric acid pressure leaching of nickeliferous limonites. Metall Trans B 8(3):547–554. https://doi.org/10.1007/BF02658621
Rubisov D, Krowinkel J, Papangelakis V (2000) Sulphuric acid pressure leaching of laterites—universal kinetics of nickel dissolution for limonites and limonitic/saprolitic blends. Hydrometallurgy 58(1):1–11. https://doi.org/10.1016/S0304-386X(00)00094-3
Georgiou D, Papangelakis V (2004) Characterization of limonitic laterite and solids during sulfuric acid pressure leaching using transmission electron microscopy. Miner Eng 17(3):461–463. https://doi.org/10.1016/j.mineng.2003.10.015
Levenspiel O (1998) Chemical reaction engineering. Wiley, Hoboken
Dreisinger D, Abed N (2002) A fundamental study of the reductive leaching of chalcopyrite using metallic iron part I: kinetic analysis. Hydrometallurgy 66(1–3):37–57. https://doi.org/10.1016/S0304-386X(02)00079-8
Nozari I, Azizi A (2020) Experimental and kinetic modeling investigation of copper dissolution process from an Iranian mixed oxide/sulfide copper ore. J Sustain Metall 6(3):437–450. https://doi.org/10.1007/s40831-020-00291-6
Liddell KC (2005) Shrinking core models in hydrometallurgy: what students are not being told about the pseudo-steady approximation. Hydrometallurgy 79(1–2):62–68. https://doi.org/10.1016/j.hydromet.2003.07.011
Wang J, Xie F, Pan Y, Wang W (2022) Leaching of gold with copper-citrate-thiosulfate solutions. Min Proc Ext Met Rev 43(7):916–925. https://doi.org/10.1080/08827508.2021.1969389
Ji G, Liao Y, Xi J, Liu Q, Wu Y, Ma H, Li J (2023) Behavior and kinetics of copper during oxygen pressure leaching of complex chalcopyrite without acid. J Sustain Metall 9:350–362. https://doi.org/10.1007/s40831-023-00658-5
Avrami M (1939) Kinetics of phase change. I General theory J Chem Phys 7(12):1103–1112. https://doi.org/10.1063/1.1750380
Avrami M (1940) Kinetics of phase change: II transformation-time relations for random distribution of nuclei. J Chem Phys 8(2):212–224. https://doi.org/10.1063/1.1750631
Avrami M (1941) Granulation, phase change, and microstructure kinetics of phase change III. J Chem Phys 9(2):177–184. https://doi.org/10.1063/1.1750872
Okur H, Tekin T, Ozer AK, Bayramoglu M (2002) Effect of ultrasound on the dissolution of colemanite in H2SO4. Hydrometallurgy 67(1–3):79–86. https://doi.org/10.1016/S0304-386X(02)00137-8
Demirkıran N, Künkül A (2007) Dissolution kinetics of ulexite in perchloric acid solutions. Int J Miner Process 83(1–2):76–80. https://doi.org/10.1016/j.minpro.2007.04.007
Acknowledgements
The work was supported by the National Natural Science Foundation (Nos. 52074136, 51974140 and 51904124), Jiangxi Provincial Cultivation Program for Academic and Technical Leaders of Major Subjects (Nos. 20212ACB204015 and 20212BCJL23052), the Jiangxi Provincial Department of Education Science and Technology Research Project (Nos. GJJ210875 and GJJ210834), the Jiangxi University of Science and Technology Landing Project (Nos. 2020033 and 2021027), and the Distinguished Professor Program of Jinggang Scholars in institutions of higher learning, Jiangxi Province.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
The contributing editor for this article was Zhongwei Zhao.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Liu, Y., Lu, Y. et al. Leaching Behaviors of Yulong Refractory Oxide Copper Ores from Tibet in Sulfuric Acid Solutions. J. Sustain. Metall. 9, 982–998 (2023). https://doi.org/10.1007/s40831-023-00700-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-023-00700-6