Abstract
An innovative approach of combining membrane and zero brine technologies for a joint treatment of industrial liquid waste is investigated regarding its environmental impacts compared to the existing liquid waste treatment. The object of investigation is the generation of waste acid solution by a hot dip galvanizing plant in Sicily, Italy. The waste acid solution contains hydrochloric acid, iron and zinc, which makes it a hazardous waste according to EU classifications. Environmental impacts are studied for two scenarios in the Tecnozinco hot-dip galvanizing plant in Sicily, Italy: (i) the current process of pickling with linear disposal of waste acid and (ii) the pickling combined with in-situ treatment of the waste acid using a combination of diffusion dialysis (DD), membrane distillation (MD) and a precipitation reactor. Results are obtained via an attributional life cycle assessment (LCA) approach focusing on the water footprint profile of the process. The linear disposal path creates significant costs, environmental burdens and risks during the 1500 km transport of hazardous liquid waste. The combination of DD and MD, complemented with a zero-brine precipitation reactor, closes internal material loops, could save local water resources and reduces costs as well as environmental impacts. Reduction potentials of 70–80% regarding most LCA impact categories can be expected for the application of the novel technology combination supporting the galvanizing pre-treatment process under study. Therefore, the application of such technology on the way forward to a more circular economy is recommended from an environmental viewpoint, especially in process plants similar to the investigated one.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last decade, Europe has experienced extremely hot summers. The 2015 summer in parts of Europe broke temperature records from over 60 years [1]. Moreover, according to the World Meteorological Organization, 2015 and the following years until 2020 were the warmest years globally since temperature was recorded [2]. As observed in Europe in 2015 and preceding years, high temperatures and lack of precipitation can contribute to droughts, which can in turn impact public water supplies and lead to economic losses [3]. In Europe, up to 40% of water use could be reduced significantly with technological improvements, thus decreasing the possibility of water shortages [4]. The implementation of those technical improvements is included in the EU circular economy action plan European Commission [5] inter alia supporting the replacement of primary materials by using secondary materials and favouring internal recycling in production plants.
As part of the European project “Resource recovery from industrial waste water by cutting edge membrane technologies” (ReWaCEM), the hot-dip galvanizing plant Tecnozinco SrL in Carini, on the island of Sicily in the southernmost part of Italy, was investigated regarding its liquid waste reduction potential originating from pickling steel products. In the framework of ReWaCEM, a full-scale internal recycling loop was established via a prototype unit and its potential for waste acid reduction tested on site [6]. To evaluate the environmental aspects of the new technology and its potential to improve the overall environmental performance of the galvanizing process, here we conduct a first life cycle assessment (LCA) of a potential annual operation at Tecnozinco, focusing on water-related characterization factors. The environmental relevance of the process is illustrated below.
Environmental Relevance of the Galvanizing Process
The metal industry is one of the largest consumers of energy and water and the producers of waste [7]. As part of metal processing, methods for surface protection such as the hot–dip galvanizing process are common. The actual hot-dip galvanizing process at Tecnozinco requires pre-treatment upstream, which can be divided into three steps: (1) degreasing, to remove oil or grease from the surface that is to be worked on; (2) acid pickling to remove iron oxides such as rust or scale (HCl is used in this case-study); (3) fluxing to activate the surface and promote reaction with zinc [8]. The second step is the most intensive regarding material consumption, and thus the environmental footprint. It consists in immersing metal pieces in HCl baths. Iron oxide is transformed to iron chloride thanks to the action of the HCl. In particular, at Tecnozinco there are seven baths at different acid concentrations which can be grouped in three classes according to their pickling effectiveness: highly, intermediate and poorly effective [9]. Pickling with acids removes non-metallic substances from the surface, such as rust and scale. This makes it possible to provide the material of required purity [10] During operation, the quality of the pickling solution constantly decreases and, considering the acid and iron concentrations, the pickling solution of the baths is “corrected” by spilling part of the solution and then refilling with water and HCl or fresh HCl make-up to remain close to the optimal operating conditions. At the end, when concentrations of acid are too low and those of iron too high, the solution must be disposed as hazardous acidic liquid waste. Considering European and national standards, regeneration of spent pickling solutions is a crucial issue regarding both the environmental protection and the economy of the process [10].
In the hot-dip galvanizing bath of the plant under study, zinc is used as the galvanizing agent, serving as protective cover of the metal pieces. The waste acid produced in the process is classified as hazardous waste under the code 06 01 02* according to the EU classification of waste [11] and contains the heavy metals zinc and iron (together up to 170 kg/m3). For a detailed description of the waste acid solution’s chemistry, see [12]. The current waste acid treatment is being considered as the “linear” process in this work, described in the following. Currently, the waste acid solution is being disposed in northern Italy. The treatment there is an energy intensive evaporation process, increasing the environmental burden by the use of fossil energy. Subsequently, the remaining acid sludge needs to be disposed underground which increases the environmental burden further. The recovered acid is of low quality and thus cannot be used for the same application. The road transport of hazardous liquid waste for approximately 1500 km to the treatment plant adds additional environmental burden and costs, also provoking the risk of critical accidents. Therefore, the reduction of waste acid solution on site before final disposal was exptected to reduce the environmental impacts caused by the overall process of surface treatment, along with the linked costs.
The reduction of waste acid can be achieved by a recovery of the spent solution to close material loops. Therefore, an innovative process combining the technologies of diffusion dialysis, membrane distillation, and a reactive precipitation stage was developed. This combination will be called the “circular” approach in the following, as it uses loops to reuse valuable outputs of a process. The feasibility of the innovative technology was tested by installing a prototype unit at Tecnozinco industrial site. For technical details of the prototype operation the reader is kindly referred to [6]. There are other processes for acid or metal recovery from pickling solutions as well, which do not make use of membrane technology but directly precipitate metals from the solution or evaporate the acids. While they are more common in practice, they are not the best available technologies because they allow for less circularity or are energy-intensive. Membrane-based technologies could replace some of these technologies in the long run [13].
The Hot-Dip Galvanizing Process from LCA Perspective
In this study, an LCA is performed to compare the linear and the circular operation at Tecnozinco. The results of the LCA and a water footprint analysis will be presented to assess the potential of the technology implementation on the environmental impact and the reduction of water consumption.
Only few LCA studies on hot-dip galvanizing processes could be identified by the authors. Relevant LCA publications focusing on metal surface treatment are [14, 15]. As the first one investigates a different surface treatment technique, namely chromic acid anodizing, it seems that there are no direct conclusions relevant to the galvanizing process except of the general recommendations to increase pickling bath lifetime and reduce energy and water consumption. The latter publication on the life cycle assessment of treating hot-dip galvanizing waste acid with solvent extraction and electrowinning showed notable environmental gains by extracting metal contaminants from the solution on site. However, the system boundaries were set much tighter around the waste acid processing and disposal instead of the entire galvanizing process, which does not allow estimates of the overall relevance of the change in waste acid treatment to the galvanizing process. The authors could not identify LCA studies on the overall hot-dip galvanizing process providing significant detail on waste acid handling. [16, 17] modelled the waste acid disposal as physicochemical treatment with subsequent landfilling of generated sludge, but do not address sludge or treatment modelling in any further detail.
Operation Principle of the Circular Waste Acid Treatment
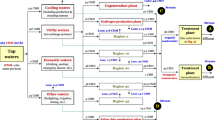
To close process loops and reduce the amount of hazardous waste generated, an innovative combination of two proven membrane technologies with a precipitation reactor was developed and applied in the pickling process of the steel processing plant. Figure 1 illustrates the previous linear operation mode as well as the developed circular operation including the new technology. While the process steps of the pre-treatment are identical in both operation modes, some in-and outputs of the baths differ due to the difference in wastewater treatment.
The innovative technology enabling a circular process combines diffusion dialysis and membrane distillation in one treatment unit, followed by a continuously stirred tank reactor (CSTR) where a reactive precipitation process occurs. The recovery of process materials for an in-house closed loop and the production of marketable iron salt are outcomes of the technology implementation. The amount of waste produced is below 10% of the previous state. The following sections briefly explains the working principles of the two membrane technologies and of the reactive precipitation.
Diffusion dialysis (DD) is a simple process with the advantage of low-energy consumption, where an ion-exchange membrane (IEM) separates two channels with solutions at different concentrations [18]. One compartment is filled with waste solution, while the other one is filled with fresh water as draw solution [19]. If an anion exchange membrane (AEM) is used, anions can diffuse through the membrane, whereas cations transport is prevented. However, considering the case of the HCl acidic pickling solution, besides the chloride anions, also protons can pass through the membrane due to their very small dimension and the tunnelling mechanism [20]. The use of the DD process for the recovery of HCl from solutions with very high concentrations of iron as in real pickling baths was studied by some of the co-authors [12, 21].
Membrane distillation (MD) is a separation process governed by thermal gradient, and thus by partial pressure difference. It means that if two channels, separated by a porous hydrophobic membrane, are at different temperatures, the passage of steam occurs from the hot side to the cool side, denying that of water due to the liquids surface tension and the hydrophobic nature of the membrane. Metal salts dissolved in the feed solution are non-volatile compounds hence massive rejection is achievable. Thus, an acid and metals concentrated solution and a slightly acidic solution (acid contaminated water) can be separated [22]. One of the biggest advantages of this technology is that the process can be performed at a feed temperature considerably lower than the liquid’s boiling point, thus allowing the use of waste heat or alternative energy sources [23]. The MD modules used in the technology proposed have a conceptually modified design in order to suit the harsh conditions of the waste pickling solutions [24].
Finally, a step of reactive precipitation was used to recover iron(III) hydroxide by adding ammonium hydroxide (28–30% w/w) as alkaline reactant. It was selected as the more suitable technology to be included in the innovative integrated process with the aim of metals separation. As iron(II) is present in the real pickling solution, also hydrogen peroxide (30% w/w) is added to oxidize iron(II) to iron(III) [25]. The result is the production of two valuable products: iron(III) hydroxide and a stream of zinc/ammonium chloride solution which can be used in the fluxing baths within the hot-dip galvanizing plant. Indeed, using this circular approach, it is possible to have (i) the continuous regeneration of the pickling solutions increasing the pickling rate and process performance, (ii) the recovery of valuable compounds (e.g., acid and metals) and (iii) a dramatic decrease of the industrial wastewater disposal [9]. A suitable use for the iron(III) hydroxide is the pigment production industry, which the product can be sold to and hence reduces the demand for it from production from the Laux-process.
Life Cycle Assessment
The comparison of the environmental impacts of both operation scenarios is follows the methodology of LCA according to the standards ISO 14040 [26] and 14044 [27], and the establishment of a water footprint profile according to ISO 14046 [28]. Water footprints build on the same fundamental framework as LCA. Both methodologies follow the physical-technological life cycle of the product or process in data collection, modelling and assessment of “the compilation and evaluation of the inputs, outputs and the environmental impacts of a product system throughout its life cycle.” [26].
For the LCA and water footprint, the GaBi Professional ts software and database system is used (in the following named GaBi), including all extensions available. The most recent available service pack version at the time of the study was 2019.3 [29].
Goal and Scope
The aim of the technology implementation analysed in this publication is the decrease in production of highly contaminated wastewater and the reduction of freshwater use, energy consumption and the use of primary materials. The success in achieving this goal will be examined here. Therefore, the goal of this study is to compare the potential environmental impacts of the linear and the circular mode of operation. The environmental impacts of the circular approach will be determined in a simulation of its full-scale implementation within the state of operation, substituting the linear operation.
Selection of Impact Categories
The environmental impact of a product system is illustrated with a selection of impact categories suitable to the goal and scope of the analysis [30]. In this study, impacts are determined for the categories listed in Table 1.
Results directly related to water are displayed in the water footprint profile, while results without direct relation to water are presented as part of the LCA results. The water footprint profile includes the effect on water scarcity according to the AWARE methodology [31]. Moreover, potential changes in water quality are assessed regarding the aquatic eutrophication on marine and freshwater ecosystems as well as the acidification. The further LCA results are presented in the impact categories climate change, terrestrial eutrophication and resource use (energy carriers).
The impact categories for both assessments were selected from the methods recommended by the Joint Research Council of the European Commission in the framework of the Environmental Footprint EF 3.0 [32], as this study was embedded in a larger European project. The impact categories in the PEF are understood to be selected by experts in the field as the best classification and characterization models available.
System Boundary and Functional Unit
The main function of the investigated process is the surface treatment of steel products in the processing line. Along the processing line, the waste acid solution accumulates and must be treated and disposed. In the circular operation part of the wastewater can be recirculated and directly used. To assess the environmental potential of the circular compared to the previous linear operation, consideration of the entire system influenced is important. This means that a common functional unit must be found that is tied to a fixed parameter defining the overall galvanization process. Therefore, the definition of the Functional Unit is:
“The treatment of waste acid solution produced by the processing of 1000 kg of steel.”
The investigated system is defined within the system boundary. The processed metal piece is prepared for the pickling process by a degreasing and stripping process, which requires thermal energy and a degreasing solution as inputs. The system boundary is visualized in Fig. 1 and its process flow further described in the following.
The pickling process itself includes a step of fresh HCl make-up at 34% w/w. As the acid is consumed by dissolving iron oxides into the solution, some fresh acid, at a standard concentration, is added as make-up to compensate the loss of acidity during the pickling process (as described in the Introduction). The water for the solution is gained from the rinsing step, which follows the pickling. After rinsing, the metal pieces enter the fluxing process, which requires an input of thermal energy. An ammonia zinc chloride solution, called “double salt”, is used as fluxing agent. Moreover, the processing chain is characterized by an overall demand on electrical energy for pumping, air pressure and the bridge crane moving the steel items. All required thermal energy is waste heat supplied from the hot-dip galvanizing process. The amount of waste heat available is significantly higher than the demand and cannot be used somewhere else. This specific situation leads to a burden-free allocation of the waste heat. During the entire process, a certain share of gaseous HCl and water vapour are emitted to air and, therefore, lost for the process.
The linear operation ends with the evaporation process to recover some acid and the final disposal of hazardous sludge in underground storage. The innovative circular technology replaces the previous linear transport to Northern Italy with an in situ treatment unit and recycles free acid from the Waste Acid Solution (WAS) back to the pickling process. The remaining solution is further treated and split into two valuable and highly pure products: iron(III) hydroxide and a substitute for the fluxing solution [25].
Both the linear and the circular operation result in a recovery of acid, but in different qualities and quantities (see Tables 3 and 4). In line with the avoided burden allocation, a credit is given in both operation modes, for the substitution of primary production.
The avoided burden allocation is applied for all marketable outputs, in both the linear and the circular operation. These outputs are recovered HCl and iron(III) hydroxide.
For the use of waste heat, a different allocation is used due to the specific situation described before.
Life Cycle Inventory
The investigated Tecnozinco hot-dip galvanizing plant has a capacity of 20,000 tons of treated steel per year. Its seven pickling baths contain slightly more than 350 m3 of acid pickling solution and consume approximately 160 to 240 tons of acid per year [9]. The annual amount of produced waste acid solution is approximately 250 tons. Referring to the data available in literature for the hot-dip galvanizing industry in Italy, similar to the European average, Tecnozinco can be classified as a small plant [33]. Independent of the plant size,general values for fresh acid consumption of 10–30 kg/ton of treated steel and for the spent liquor production of 15–45 kg/ton of treated steel are reported by AIZ (The Italian Galvanizing Association) [34].
In the following, we present the inventory data for both product systems. The reference for the process data is “1 year of operation” at the galvanizing plant. The data is obtained as one average reference year from production data of 5 years (2011–2015). A detailed LCI model was derived containing all input and output flows of the investigated product systems. In order to facilitate an understanding of the presented in- and output flows, two separate life cycle inventories for current state and future state are provided. The data origin is indicated in the LCI Tables 3 and 4 and abbreviated as given in Table 2. While all thermal energy is provided directly by waste heat from the galvanizing process, the electrical energy is drawn from the Italian grid and modelled accordingly.
For the data collection and LCA model development, several assumptions were necessary. A comprehensive description of the assumptions and the related limitations of the study are provided in chapter 7.
The input parameters only change slightly between the linear and the circular operation due to an improved process. The major difference is in the output parameters, as seen in Tables 3 and 4: In the linear state, 33.3 kg/t are sent for disposal, as in the circular state a circulating flow of 52.7 kg/t is sent to regeneration and further treatment for recovery of secondary materials. The operating materials are increasing the input substances in the circular operation, but as shown in chapter 5, it does not add relevant environmental burden on the overall process.
A detailed explanation on how the in- and outputs are modelled in GaBi software is provided in chapter 7, Assumptions and limitations.
Results
The results from the LCA and water footprint profile are presented in Table 5. The circular operation reduces the estimated environmental impact throughout all analysed impact categories. Enormous reductions of 70% and more can be observed for five of the analysed seven impact categories: climate change, marine and terrestrial eutrophication, acidification, and resource use of energy carriers.
The results per impact category are composed of contributions from various process steps. A detailed analysis of the results is provided in the following subchapters. The strong reductions in Table 5 are caused by the drastic change in waste acid treatment. While the linear operation causes a high environmental impact, the circular operation results in the generation of secondary HCl and iron(III) hydroxide avoiding primary production of those materials. These are included in the balance as negative contributions (“credits”), leading to a lower overall impact in the circular operation through the avoidance of primary material provision by producing substitute material. The largest credit is caused by the production of iron(III) hydroxide, while the recovery of HCl has rather a small impact.
Since this credit has a major effect on the results, it is further investigated in a sensitivity analysis in chapter 6.
Water Footprint Profile
The water footprint profile comprises the change in water quality as well as effects on water scarcity. The full results for linear and circular operation are illustrated broken down into the major process steps as shown in Fig. 2. All categories show a reduced impact in the circular operation. This is mostly an effect of the change in waste acid treatment, which in linear operation for acidification and marine eutrophication contributes the most. Waste acid treatment set aside, the consumption of hydrochloric acid contributes significantly to the impact of both product systems, especially via freshwater eutrophication. The electrical energy consumption further contributes a relevant share to all categories, especially water scarcity and eutrophication. This is due to upstream water use in gas, oil and coal provision. Over 50% of the operation’s water scarcity related water use is caused by indirect water use during the electricity production, spread over several processes including the evaporation in the linear disposal route. Nevertheless, the circular operation reduces the water use by about 50% due to the less energy intensive waste acid treatment and the recovery of process materials in the reactive precipitation. HCl production as well as the process energy differ only slightly between both operation modes.
The main reason for the overall reduced impact of the waste acid treatment is the recovery of valuable substances instead of the previous disposal route. The production of iron(III) hydroxide from the reactive precipitation avoids the conventional route via the Laux process. This process was selected as avoided reference production route, as among the several potential applications for the iron(III) hydroxide, the use in pigment production is common and competitive. The avoided burden of primary production in the circular operation leads to a negative impact in acidification and water scarcity, as well as in marine eutrophication.
LCA Results
Figure 3 illustrates the LCA results, where the contribution of single processes to the respective total of climate change, terrestrial eutrophication and the resource use of energy carriers show a ranking similar to the water footprint profile.
The major contribution to the greenhouse gas (GHG) emissions assessed in the climate change category originates from the disposal route in linear operation. The truck transport of the waste acid and the final underground disposal of hazardous sludge is the main source of GHG resulting from the linear waste acid treatment process. Therefore, the improvement with the circular approach is very large with a reduction of about 72%. The effect is similar for terrestrial eutrophication and the resource use of energy carriers. While the waste acid treatment contributes the highest share of environmental burden in linear operation, the circular approach leads to a recovery of iron(III) hydroxide from the reactive precipitation, substituting primary materials [33]. A detailed description of this process can be found in Randazzo et al. [25]. It contributes a credit to the balance and is represented as negative values in Fig. 3. The remaining environmental impact originates from the process electricity and the production of the HCl.
Sensitivity Analysis—Influence of Credit on Results
The study presented in the previous chapters shows the intended implementation of the developed technology, resulting in the production of marketable secondary resources instead of waste for disposal.
The results presented in chapter 5 show how essential it is to produce a sellable quality, because this allows a credit to be applied as part of the avoided burden allocation. To investigate the sensitivity of the results to the applied credits, the LCA model is calculated additionally without any credit given. The results are provided in Table 6.
The results of the sensitivity analysis show that the linear operation has an increased environmental impact between 6% for water scarcity and 11% for resource use (energy carriers), if no credit is given for the recovery of HCl by evaporation. For the circular operation an increased impact between 21% for water scarcity and 79% for resource use (energy carriers) is calculated for the process without a credit from iron(III) hydroxide. The interpretation of these results is that the circular operation is still superior to the linear one. Even without the credits, the benefits of the circular operation outweigh the linear operation clearly.
Assumptions and Limitations
As defined in the section on goal and scope of the study, the present study focuses on the analysis of relevant environmental impact categories and water assessment. Since the investigated membrane technologies are currently still under development, predictions for their final application cannot be made with certainty. Therefore, the calculated linear upscaling of the technology might not depict the environmental impacts of real full-scale applications accurately.
The investigated process line is the pre-treatment of a hot dip galvanizing process. The actual galvanizing process is not part of the study since it is not affected by the new technology. Furthermore, the treated steel is not included in the study, due to the high variability of products being treated.
The presented study is based on calculations and assumption where primary data were not available. This applies primarily to mass flow data within the system as well as gaseous emissions. Furthermore, the origin of the operating materials could not be consistently determined, hence the dominating market share was used for the LCA model. The database processes used, are selected according to their availability as close as possible to the local conditions. In some cases, European or global average values are used.
The foreground system is based on primary data collection and expert interviews of involved plant engineers and researchers. Upstream information is collected from Tecnozinco staff as far as possible and complemented with data from GaBi database.
Chemical processes in the pickling and fluxing as well as in both end-of-life processes are modelled according to stoichiometric reactions and must therefore been understood as ideal state. This is due to the fact, that no primary data collection was possible for the reactions itself. The authors are well aware of the fact, that reality is not an ideal state and advise the reader to be aware of that as well. The stoichiometric calculations as well as other assumptions are taken as close to the reality expected by the experts in the field. We expect the trend of the results to remain the same as in ideal state.
The underground storage of hazardous acidic sludge contributes a significant amount to the environmental impact of the linear operation and is based on generic GaBi datasets due to the lack of other available information. A different disposal might significantly affect the results, and therefore, we highlight that the results from this study are not to be interpreted generically but always in the context of this particular system boundary assessed.
The water scarcity assessment based on AWARE characterisation factors is a young impact assessment method and still in refinement and further development. As such, the results must be understood as proof of method application and ballpark figure but not as highly precise quantitative result.
In the specific case of the Tecnozinco plant, the hot-dip galvanizing process provides much more waste heat than the neighbourhood can use. Thus, the whole pre-treatment under study in this research paper is supplied with waste heat and regarded as burden-free. This only applies to applications with the same freely available waste heat and cannot be understood as standard case.
The thermal energy is modelled as burden free, since waste heat is used which does not have any other available purpose.
The degreasing solution is a mix of hydrochloric and phosphoric acid and is modelled with German production data from GaBi database, as well as the hydrochloric acid for the pickling. Different production routes will effect the related environmental impact, especially the electricity source for the production has a strong effect on the CO2-emissions.
For the tap water the generic Italian tap water from groundwater process is modified with regionalized scarcity factors for Sicily. The applied AWARE factors are taken from the most recent WULCA publication [31].
The production of double salt is modelled as stoichometric combination from ammonium chloride and zinc chloride from GaBi database. Additional process energy is neglected as minor relevant.
The transport process of the waste disposal in the linear operation is calculated with a 32 t diesel truck.
Gaseous HCl water vapour emissions are included in the GaBi model as emission flows from the database. The final sludge disposal is modelled with a generic dataset for acid/basic sludge treatment through neutralisation, incineration, microencapsulaton and macroencapsulation for underground storage.
The recovered acid is modelled with a credit given with the identical dataset process which is used as input, scaled down to the concentration of the recovered acid gained.
For the circular operation, additionally the inputs of ammonium hydroxide and hydrogen peroxide are modelled in their used concentration (30%), based on a mix from 100% and deionised water. The produced iron(III) hydroxide in the reactive precipitator is modelled as avoided burden of the production of the same substance via the Laux-process. The therefore required iron, nitrobenzene and water are modelled with GaBi database from Germany and EU as best available data. The simultaneosly produced Anilin is neither included in the balance as burden nor as credit.
As described in the LCA standards ISO 14040 and 14044, the results are only applicable for the given system boundary and the data applied. Therefore the main limiting factor of this study is set by the system boundary described in chapter 3. Further investigation on the technology including a permanent monitoring of the installed pilot plant is recommended in order to validate the results and the promising circular approach.
Discussion, Conclusion and Outlook
The presented study analyses the process adaptation of a hot-dip galvanizing plant in Sicily, Italy on its environmental impacts with LCA and a water footprint profile. The analyses provide environmental potentials of the innovative technology approach integrated into an existing linear production of steel products. Overall, the environmental impacts of the linear operation are dominated by the linear disposal route of including a long distance transport and underground final storage of hazardous sludge.
This existing waste acid treatment in the linear operation is based on the transport of hazardous waste from Sicily to northern Italy and has a strong contribution to climate change relevant emissions as well as to eutrophication and acidification potential. Therefore, a significant improvement can be achieved by the process adaptation to a circular operation, by around 75% in five of the seven analysed impact categories. Over 50% of the operation’s scarcity related water use is caused by indirect water use during the electricity production. The circular operation reduces that water use by about 50% due to the less energy intensive waste acid treatment and the recovery of process materials.
The most sensitive parameters of the LCA model are identified with the credit given for the iron(III) hydroxide and the thermal energy provision via waste heat.
In further studies it is recommended to investigate varying scenarios on the potentially equal usability and marketability of the secondary material. Moreover, a different avoided production route or varying allocation in the iron(III) hydroxide production should be studied. Nevertheless, with a smaller value the environmental benefit might decrease in the LCA, but the technology is still expected to be profitable for both the environment and economically for the operator.
For the applied heat source, further studies are also recommended. The use of waste heat is strongly advised as the authors expect the use of gas or electricity as heat source to increase the environmental burden significantly, making the benefit disappear completely. In case of lacking waste heat, solar thermal heaters are recommended as thermal energy source for the operation of the MD.
Further development potential was already identified by the local research group at the University of Palermo to target a direct water use of zero, with an additional MD module to recover process water. Since it is not technically applied yet, further research on the topic is necessary and promising, given the environmental potentials identified by this study.
As this is probably the first LCA and water footprint study concerning the pre-treatment steps for hot-dip galvanizing in such detail, we are happy to contribute in filling this gap. We hope that further studies will confirm our results and encourage scientists to perform similar studies.
Overall, the LCA as well as the water footprint profile of the technology implementation show very promising improvements to close loops in an existing industrial linear production line. The combination of different membrane systems and zero brine technology has proven its functionality and individual adjustment flexibility to a specific application. A further roll-out of the technology in more industrial production lines is recommended in support of environmental impact reduction following the Sustainable Development Goals of the United Nations and the EU Green Deal. Furthermore, the change to a renewable energy source can contribute to a reduction of the environmental impact, especially for the overall process including the energy intensive preheating and hot-dip galvanisation itself. Due to the high potential of solar and wind power in Sicily, it is strongly recommended to actively work towards the goals of the EU green deal.
Data Availability
Data collection from University of Stuttgart and University of Palermo in direct contact with Tecnozinco. Verification of models and assumptions by University of Stuttgart with University of Palermo. All supporting background data for the LCA analysis are the property of the Sphera GaBi Database and software.
References
Hanel M, Rakovec O, Markonis Y (2018) Revisiting the recent European droughts from a long-term perspective. Sci Rep 8:8499
World Meteorological Organization (2022) State of the Climate in Europe 2021, WMO-No. 1304
Ionita M et al (2015) The European 2015 drought from a climatological perspective. Hydrol Earth Syst Sci 21(2017):1392–1419
European Commission (2011) Fahrplan für ein ressourcenschonendes Europa. European Commission, Brüssel
European Commission (2020) A new circular economy action plan for a cleaner and more competitive Europe. European Commission, Brussels
Gueccia R, Winter D, Randazzo S, Cipollina A, Koschikowski J, Micale G (2021) An integrated approach for the HCl and metals recovery from waste pickling solutions: pilot plant and design operations. Chem Eng Res Des 168:383–396
Panigrahi J, Sharma SC (2014) Simulation, control and optimization of water systems in industrial plants. In: Ranade VV, Bhandari VM (eds) Industrial wastewater treatment, recycling and reuse. Butterworth-Heinemann, Oxford, pp 463–487
Stocks C, Wood J, Guy S (2005) Minimisation and recycling of spent acid wastes from galvanizing plants. Resour Conserv Recycl 44:153–166
Culcasi A, Gueccia R, Randazzo S, Cipollina A, Micale G (2019) Design of a novel membrane-integrated waste acid recovery process. Clean Prod. https://doi.org/10.1016/j.jclepro.2019.117623
Regel-Rosocka M, Cieszynska A, Wisniewski M (2007) Methods of regeneration of spent pickling solutions from steel treatment plants. Pol J Chem Technol 9(2):42–45
European Commission (2018) Commission notice on technical guidance on the classification of waste. Official Journal of th European Union C124
Gueccia R, Ruiz Aguirre A, Randazzo S, Cipollina A, Micale G (2020) Diffusion dialysis for separation of hydrochloric acid, iron and zinc ions from highly concentrated pickling solutions. Membranes 10:129–145
Regel-Rosocka M (2010) A review on methods of regeneration of spent pickling solutions from steel processing. J Hazard Mater 177:57–69
Harscoet E, Froelich D (2008) Use of LCA to evaluate the environmental benefits of substituting chromic acid anodizing (CAA). J Clean Prod 16:1294–1305
Arguillarena A, Margallo M, Irabien Á, Urtiaga A (2022) Life cycle assessment of zinc and iron recovery from spent pickling acids by membrane-based solvent extraction and electrowinning. J Environ Manage 318:1–9
Arguillarena A, Margallo M, Urtiaga A (2021) Carbon footprint of the hot-dip galvanisation process using a life cycle assessment approach. Cleaner Eng Technol 2:1–8
Arguillarena A, Margallo M, Urtiaga A, Irabien Á (2021) Life-cycle assessment as a tool to evaluate the environmental impact of hot-dip galvanisation. J Clean Prod 290:1–11
Luo J, Wu C, Xu T, Wu Y (2011) Diffusion dialysis-concept, principle and application. J Membr Sci 366:1–16. https://doi.org/10.1016/j.memsci.2010.10.028
Mao F, Zhang G, Tong J, Tongwen Xu, Yonghui Wu (2014) Anion exchange membranes used in diffusion dialysis for acid recovery. Sep Purif Technol 122:376–383
Strathmann H (2004) Electrochemical and thermodynamic fundamentals. In: Strathmann H (ed) Ion-exchange membrane separation processes. Elsevier, Amsterdam, pp 23–88
Gueccia R, Randazzo S, Chillura Martino D, Cipollina A, Micale G (2019) Experimental investigation and modeling of diffusion dialysis for HCl recovery from waste pickling solution. J Environ Manag 235:202–212
Alkhudhiri A, Darwish N, Hilal N (2012) Membrane distillation: a comprehensive review. Desalination 287:2–18
Tomaszewska M, Gryta M, Morawski AW (2001) Recovery of hydrochloric acid from metal pickling solutions by membrane distillation. Sep Purif Technol 22–23:591–600
Schwantes R et al (2019) Characterization and assessment of a novel plate and frame MD module for single pass wastewater concentration-FEED gap air gap membrane distillation. Membranes 9(9):118
Randazzo S, La Corte D, Gueccia R, Cipollina A, Micale G (2021) Metals recovery from waste pickling solutions by reactive precipitation. Chem Eng Trans 86:1045–1050
EN ISO 14040:2006 + Amd 1:2020 (2006) Umweltmanagement–Ökobilanz–Grundsätze und Rahmenbedingungen. Beuth-Verlag, Berlin
EN ISO 14044:2006 + Amd 1:2017 + Amd 2:2020 (2006) Umweltmanagement–Ökobilanz–Anforderungen und Anleitungen. Beuth-Verlag, Berlin
EN ISO 14046:2016 (2016) Umweltmanagement–Wasser-Fußabdruck–Grundsätze, Anforderungen und Leitlinien. Beuth-Verlag, Berlin
Sphera Solutions GmbH (2019) GaBi Professional ts
Rosenbaum RK, Hauschild MZ, Olsen S (2018) Life cycle impact assessment. In: Hauschild MZ et al (eds) Life cycle assessment—theory and practice. Springer, Cham
Boulay AM et al (2018) The WULCA consensus characterization model for water scarcity footprints: assessing impacts of water consumption based on available water remaining (AWARE). Int J Life Cycle Assess 23:368–378
Fazio S, Biganzioli F, de Laurentiis V, Zampori L, Sala S, Diaconu E (2018) Supporting information to the characterisation factors of recommended EF life cycle impact assessment methods, version 2, from ILCD to EF 3.0. European Commission, Ispra
Gueccia R et al (2022) Economics and environmental benefits of waste pickling solution valorization. Membranes 12:114–133
Pernice L (2017) Personal communication. Associazione Italiana Zincatura (AIZ), Vienna
European Commission (2017) PEFCR Guidance document—guidance for the development of Product Environmental Footprint Category Rules (PEFCRs), version 6.3, December 2017. European Commission, Brussels
Jaab S, Beusen A, Huijbregts H, van Jaarsveld MAJ (2009) Chapter 6: Eutrophication. In: Goedkoop M, Heijungs R, Huijbregts M, Schryver A, Struijs J, Zelm R (eds) ReCiPe 2008: a life cycle impact assessment method which comprises harmonised category indicators at the midpoint and the endpoint level. Ministerie van VROM, Den Haag, pp 59–66
Jolliet O, Antón A, Boulay AM, Cherubini F, Fantke P (2018) Global guidance on environmental life cycle impact assessment indicators: impacts of climate change, fine particulate matter formation, water consumption and land use. Int J Life Cycle Assess 23:2189–2207
Myhre G et al (2013) Anthropogenic and natural radiative forcing. In: Stocker TF, Qin D, Plattner GK and Allen SK, Tignor M (eds) Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, United Kingdom, Cambridge University Press
van Oers L, de Koning A, Guinée JB, Huppes G (2002) Abiotic resource depletion in LCA—improving characterisation factors for abiotic depletion as recommended in the new Dutch LCA handbook. Road and Hydraulic Engineering Institute, Amsterdam
Funding
Open Access funding enabled and organized by Projekt DEAL. This work is based on results of the publicly funded project ReWaCEM, kindly funded within the European Union’s Horizon 2020 research and innovation programme under Grant Agreement Number 723729.
Author information
Authors and Affiliations
Contributions
ML, GS and TMP: Conceptualization, ML, TMP and FG: methodology, ML: software, ML, SR, and RG: data collection, analysis and verification, FG and SR: supervision, ML, GS, TMP, SR, RG and FG: writing—review and editing, ML, GS and TMP: writing—original draft preparation, ML, SR and RG: investigation, FG and GS: visualization, SR and ML: project administration, SR and FG quality assurance
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Additional information
The contributing editor for this article was Christina Meskers.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lorenz, M., Seitfudem, G., Randazzo, S. et al. Combining Membrane and Zero Brine Technologies in Waste Acid Treatment for a Circular Economy in the Hot-Dip Galvanizing Industry: A Life Cycle Perspective. J. Sustain. Metall. 9, 537–549 (2023). https://doi.org/10.1007/s40831-023-00668-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-023-00668-3