Abstract
Introduction
This analysis compared healthcare resource use (HCRU) and costs associated with incident organ damage in a cohort of adult patients with systemic lupus erythematosus (SLE).
Methods
Incident SLE cases were identified (Clinical Practice Research Datalink [CPRD] and Hospital Episode Statistics-linked healthcare databases; January 1, 2005–June 30, 2019). Annual incidence of 13 organ damage domains was calculated from SLE diagnosis through follow-up. Annualized HCRU and costs were compared between organ damage and non-organ damage patient groups using generalized estimating equations.
Results
A total of 936 patients met the inclusion criteria for SLE. Mean age was 48.0 (standard deviation [SD] 15.7) years and 88% were female. Over a median follow-up period of 4.3 (interquartile range [IQR] 1.9–7.0) years, 59% (315/533) had evidence of post-SLE diagnosis incident organ damage (≥ 1 type), which was greatest for musculoskeletal (146/819 [18%]), cardiovascular (149/842 [18%]), and skin (148/856 [17%]) domains. Patients with organ damage had greater resource use for all organ systems, excluding gonadal, versus those without it. Overall, mean (SD) annualized all-cause HCRU was greater in patients with organ damage versus those without it (inpatient, 1.0 versus 0.2; outpatient, 7.3 versus 3.5; accident and emergency, 0.5 versus 0.2 days; primary care contacts, 28.7 versus 16.5; prescription medications, 62.3 versus 22.9). Adjusted mean annualized all-cause costs were significantly greater in both post- and pre-organ damage index periods for patients with organ damage versus those without it (all P < 0.05, excluding gonadal). Overall organ damage was associated with significantly increased adjusted mean annualized per-patient cost (£4442 greater [P < 0.0001]) ranging between £2709 and £7150 greater depending on the organ damage type.
Conclusion
Organ damage was associated with higher HCRU and healthcare costs, before and after SLE diagnosis. More effective SLE management may slow disease progression, prevent organ damage onset, improve clinical outcomes, and reduce healthcare costs.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Why carry out this study? |
Patients with systemic lupus erythematosus (SLE) are at increased risk for organ damage as well as greater healthcare resource use (HCRU) stemming from both long-term disease activity and corticosteroid treatment. |
While several studies have examined HCRU and direct medical costs in patients with SLE, there are relatively few publications on the direct impact of organ damage on HCRU and costs for newly diagnosed patients. |
What was learned from this study? |
Over half of patients with SLE experienced organ damage in ≥ 1 organ system following SLE diagnosis. |
The highest incidence of organ damage in patients with SLE was in musculoskeletal, cardiovascular, and skin domains. |
Patients with SLE who have organ damage had greater HCRU and costs before and after diagnosis versus in patients without organ damage. |
Per-patient treatment costs in the United Kingdom were substantial and persisted post-diagnosis for patients with SLE. |
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease with multiorgan involvement and associated high morbidity and mortality. SLE is characterized by variable clinical presentation and fluctuating disease activity, with the course of disease often having periods of low disease activity, interspersed with measurable increases in disease activity in one or more organ systems, referred to as flares. The majority (> 95%) of patients experience at least one flare post-diagnosis; such flares increase in frequency as disease severity increases. Flares can contribute to organ damage and adverse outcomes [1,2,3,4,5]. Any organ system can be affected, with renal, neuropsychiatric, pulmonary, and cardiovascular systems all commonly involved. Organ damage accrues over time and is associated with increased risks of hospitalization and death [6,7,8], with certain ethnic groups at higher risk of organ damage accrual and poorer outcomes [9, 10].
Patients with SLE have poor health-related quality of life [11,12,13] and high healthcare resource use (HCRU) [4, 14,15,16,17,18], particularly in the presence of severe or active disease, flares, and organ damage. Thus, the aim of SLE management is remission, or the attainment of the lowest possible disease activity, preventing flares, and minimizing glucocorticoid use, thereby protecting patients from organ damage, and improving clinical outcomes and quality of life [1, 19].
While several studies have examined the impact on HCRU and direct medical costs in patients with SLE [4, 15, 20], there are relatively few publications on the impact of organ damage on HCRU and costs for newly diagnosed patients. A recent study described costs associated with organ damage states across the disease course using multistate modeling [21]. This study in a multicenter, international inception cohort demonstrated the substantial increase in healthcare costs in patients with greater organ damage compared with patients with no organ damage. There are currently no longitudinal data describing HCRU and costs associated with SLE for patients with and without organ damage over time in the United Kingdom (UK).
Our study aimed to identify patients with organ damage after SLE diagnosis and to compare HCRU and costs in patients with and without organ damage in a longitudinal cohort of patients newly diagnosed with SLE in the UK using data from the linked Clinical Practice Research Datalink (CPRD) and Hospital Episode Statistics (HES) database.
Methods
Study Design
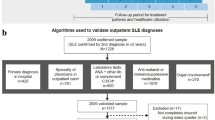
This was an observational, retrospective, longitudinal cohort study of adult patients with SLE in all four UK nations. Incident cases of SLE were identified in the CPRD and HES-linked healthcare administrative databases from January 1, 2005 to June 30, 2019 (Fig. 1A).
A Study timeline and parameters and B flowchart of study cohort. CPRD Clinical Practice Research Datalink, HCRU healthcare resource use, HES Hospital Episode Statistics, ICD International Classification of Diseases, NSAID non-steroidal anti-inflammatory drug, SLE systemic lupus erythematosus, UTS up-to-standard
Data Sources
Routinely collected data were sourced from two linked data sources in the UK: CPRD GOLD, which contains primary care data from 416 general practices and includes data on 10,800,187 patients eligible for linkage, and the HES database, which contains secondary care information (hospital admissions, accident and emergency [A&E], and the International Classification of Diseases, Tenth Revision [ICD-10]) for the coding of diagnosis and type of admission. Death registration data, used to identify mortality and therefore patient exit from the data sources, were obtained from the Office for National Statistics.
Population
All patients aged ≥ 18 years presenting to a general practitioner or hospital with ≥ 1 diagnosis of SLE during the study period were eligible for inclusion. SLE diagnosis was based on READ codes (CPRD GOLD) or ICD-10 codes (HES database), with code lists determined by a panel of clinical experts and aligned with previous CPRD studies, which are detailed in the Supplementary Material (Supplementary Material Table S1) [22].
Verification of SLE was required, based on repeat diagnosis (in CPRD or HES), rheumatologist referral/appointment, or specific medication use (corticosteroids, immunosuppressive therapy or hydroxychloroquine) according to an algorithm developed in a previous study [23]. To exclude prevalent cases, patients were required to have ≥ 12 months SLE disease-free time before the SLE diagnosis date. Patients were followed from SLE diagnosis date until the end of the study period, database exit/last observed visit, or death, whichever came first. Patients were excluded if they had READ codes indicating cutaneous, drug-induced, or discoid lupus rather than systemic lupus; they transferred out of the practice prior to the index event date (date of first eligible diagnosis); or they did not have at least 12 months of valid data prior to index diagnosis.
Study Outcomes
Incident Organ Damage
New cases of organ damage in the patient cohort were identified between SLE diagnosis date and June 30, 2019. Patients with organ damage included those with no organ damage diagnosis in the 12 months prior to the SLE diagnosis date and ≥ 1 diagnosis of organ damage at any time from SLE diagnosis date to end of follow-up. The date of first diagnosis of organ damage was assigned as the organ damage index date.
Patients without organ damage had no diagnosis of organ damage at any time from the SLE diagnosis date to end of follow-up or in the 12 months prior to the SLE diagnosis date. Patients without organ damage were assigned an index date that corresponded to the average time between first SLE diagnosis and the date of organ damage calculated among patients with organ damage.
As organ damage defined by the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI) [24,25,26,27] is not captured in the CPRD, code lists were developed as a proxy for organ damage informed by the SDI and a literature review for each organ damage condition captured in CPRD and HES for 13 organ system categories (Supplementary Material Table S2): cardiovascular, endocrine (diabetes requiring therapy), gastrointestinal, gonadal (premature failure), hepatic, malignancies (any), musculoskeletal, neuropsychiatric (including cognitive impairment), ocular, peripheral vascular, pulmonary, renal, and skin. Development of code lists involved the use of the CPRD GOLD Medical Dictionary and International Statistical Classification of Diseases and Related Health Problems 10th Revision to identify a code list for each condition. In order to identify patients with organ damage rather than other comorbidities we focused on chronic disease terms where available, apart from those defined as occurring “ever” in the SDI.
Each of the 13 organ damage categories was examined separately, meaning a single patient could be included in more than one organ damage analysis. Any patient with organ damage during follow-up was presented in a combined “all organ damage” group. The number of organ damage conditions per year was calculated starting from the SLE diagnosis date (date of first SLE diagnosis) and then for each year until the end of follow-up (June 30, 2019).
HCRU and Costs
The HCRU/cost analysis comprised only patients with a post-organ damage index date follow-up of ≥ 12 months. Inpatient care, outpatient attendances and procedures, A&E visits, primary care contacts, and prescription medication use were captured and used to estimate costs to the UK National Health Service in 2019 British pound sterling (GBP), using standard unit costing methods [28, 29]. Estimated costs included only direct healthcare costs; no indirect costs (e.g., lost work productivity, caregiver expenses) were captured.
Statistical Analyses
Patient characteristics at the time of SLE diagnosis are presented using descriptive statistics, overall and grouped according to presence or absence of organ damage after index date.
Unadjusted means and standard deviations (SD) were estimated to summarize annual counts by type of utilization for patients with and without organ damage prior to the SLE diagnosis date and for each 12-month period up to 10 years following the SLE diagnosis date. Generalized estimating equations were used to compare the difference in all-cause annual healthcare costs (inpatient stays, outpatient visits, A&E visits, primary care contacts, and primary care prescriptions) between patients with and without organ damage, for organ damage overall, and for each of the 13 organ damage categories separately.
All models were adjusted for age, gender, and time since organ damage index date. Models accounted for repeated annual measures and included all available data (each patient could have different lengths of follow-up) but excluded any incomplete years of data. All analyses were conducted using SAS software 9.4 (SAS Institute, Cary, NC, USA).
Compliance with Ethics Guidelines
This study involves anonymized patient data and does not require ethics board approval. The study was approved by the CPRD Independent Scientific Advisory Committee (ISAC protocol 17_281) on January 28, 2021.
Results
Study Cohort
From the CPRD database, 4414 patients had an SLE code (READ/ICD-10) and a patient record linked to HES identified between January 1, 2005 and June 30, 2019. Of these, 936 patients satisfied all inclusion criteria, including verification of SLE diagnosis, and were included in the overall study cohort (Fig. 1B).
The mean age at SLE diagnosis was 48.0 years and most patients (88%; n = 826) were women (Table 1). The median (interquartile range) duration of follow-up was 4.3 (1.9–7.0) years. Patients with organ damage were typically older (n = 315, mean age 47.6 years; 54% aged ≥ 45 years) than those without organ damage (n = 218, mean age 43.5 years; 44% aged ≥ 45 years). Both groups were balanced in their proportion of women, mean weight (kg), and body mass index (Table 1).
Incident Organ Damage
Overall (All Organ Damage)
Within the study cohort (N = 936), 533 patients (57%) had no diagnosed organ damage in any of the 13 categories in the 12 months prior to SLE diagnosis and were included in the “all organ damage” risk group category. Multiorgan involvement was common, with 315/533 (59%) patients having ≥ 1 type of organ damage during post-SLE diagnosis follow-up (Fig. 2; Supplementary Table S3), including 152/533 (29%) patients who had a diagnosis of organ damage within 1 year, and 284/533 (53%) who had a diagnosis of organ damage within 5 years.
Multiorgan involvement (diagnosis of organ damage in > 1 system) over the entire follow-up period was present in 30% (159/533) of patients, with 18% (97/533) of patients having a diagnosis of organ damage in ≥ 3 systems, and 6% (34/533) of patients having a diagnosis of organ damage in ≥ 5 systems.
Individual Organ Systems
To examine each organ damage category, separate risk cohorts for each analysis were constructed and patients with that specific organ damage in the 12 months prior to the SLE diagnosis date were excluded. Accordingly, of the 936 patients in the study cohort, a different number of patients were included in each organ damage category, which prevents side-by-side comparisons across some organs (Supplementary Table S3).
The highest cumulative incidence of organ damage at any time during follow-up was for the musculoskeletal domain, where out of the 819 patients who did not have organ damage at baseline, 146 patients (18%) developed organ damage during follow-up (Supplementary Material Table S3; Figure 2). Other domains with high cumulative incidence of organ damage during follow-up included the cardiovascular (149/842 [18%]) and skin (148/856 [17%]) domains, and the least commonly affected systems were hepatic (43/875 [5%]) and gonadal (17/932 [2%]).
HCRU and Costs
The distribution of organ damage by organ system in the HCRU/cost cohort, which included only patients with post-organ damage index date follow-up of ≥ 12 months, is shown in Supplementary Material Figure S1. For all types of healthcare use, patients with organ damage generally had higher resource use than the no-organ damage controls, both post- and pre-organ damage index, excluding gonadal (Table 2). In the overall (all organ damage) group, 256/378 (68%) patients had organ damage and the mean (SD) annualized all-cause HCRU was higher in patients with versus without organ damage post diagnosis (inpatient, 1.0 versus 0.2; outpatient, 7.3 versus 3.5; A&E, 0.5 versus 0.2; primary care contacts, 28.7 versus 16.5; and prescription medications, 62.3 versus 22.9), a trend also observed in the overall pre-organ damage group.
The unadjusted mean all-cause costs per patient (Supplementary Material Table S4) were up to six times higher in patients with versus without organ damage, post-organ damage index, across all types of HCRU and organ damage categories, except for the gonadal group. The main drivers of all-cause costs post-organ damage index for patients with and without organ damage were primary care contacts (£1319–4542), followed by prescriptions (£896–2592), and were highest for patients with organ damage. In the overall organ damage group, for patients with versus without organ damage, the all-cause costs post-organ damage index were highest for primary care contacts (£2727 versus £1319), followed by primary care prescriptions (£1777 versus £376), inpatient stays (£1099 versus £191) and outpatient visits (£1063 versus £464), and lowest for A&E visits (£37 versus £13). For overall HCRU, the unadjusted mean all-cause cost per patient in the overall (all organ damage) group was 2.8 times higher in patients with organ damage versus no organ damage, post-organ damage indexing.
When adjusted for age, gender, and time since organ damage index, mean all-cause costs (Fig. 3; Supplementary Material Table S5) followed the same pattern of significantly higher costs in both the post- and pre-organ damage index periods for patients with organ damage than the no-organ damage controls, in all organ domains. However, the gonadal domain was the only domain that did not have higher costs for patients with versus without organ damage. Overall, the difference in adjusted mean annualized per-patient cost estimates between patients with organ damage compared with those without organ damage was £4442 (P < 0.0001). Having an affected organ system (excluding gonadal) increased annualized per-patient cost estimates by between £2709 and £7150, post-organ damage indexing. The difference in regression-adjusted all-cause annual costs per patient attributable to organ damage in each year compared with the baseline year increased gradually over time, except for a peak in the year that organ damage was diagnosed (Fig. 4).
Regression-adjusted all-cause annual costs per patient with SLE (2019 GBP) attributable to organ damage*. Includes only patients from the overall (all organ damage) subgroup in the HCRU/costs cohort (a post-organ damage index date follow-up of ≥ 12 months) (n = 378). *Difference in difference = total costs attributable to organ damage in each year compared with the baseline year (difference in costs each year compared with baseline year for organ damage cases, minus the difference in costs compared with baseline year for no organ damage). Calculated using generalized estimating equations accounting for correlated data to compare mean all-cause healthcare costs between patients before and after organ damage index date, adjusting for age, gender, and correlated variables. CI confidence interval, GBP British pound sterling, SLE systemic lupus erythematosus
Discussion
This longitudinal, observational cohort study analyzed HCRU and costs in newly diagnosed patients with SLE in the UK undergoing treatment in real-world settings, using routinely captured hospital and primary care data on a large and representative population-based sample. More than half of patients in our study experienced incident organ damage in at least one organ system within 5 years of SLE diagnosis. Patients with incident organ damage had higher HCRU and healthcare costs compared with patients without organ damage.
This study provides novel insights into the incidence, HCRU, and all-cause healthcare costs due to organ damage in patients with SLE over time in the UK. In this analysis, 59% of newly diagnosed patients experienced organ damage during the 10-year follow-up despite receiving treatment for SLE, with the musculoskeletal, cardiovascular, and skin systems showing the highest cumulative incidences of organ damage. Previous longitudinal studies have observed accumulation of organ damage to be highest in these organ systems, in addition to renal damage, which is a key long-term manifestation that becomes more significant with SLE duration. Comorbidities linked to organ damage, such as cardiac arrythmia, have been reported to be more than twice as prevalent in patients with SLE versus the general population, with this organ damage accrual also being observed prior to SLE diagnosis [30, 31]. In our study, just over 1% of patients experienced cardiac arrythmia prior to SLE diagnosis, and 13% of patients had a Charlson comorbidity index of ≥ 1, indicating that patients in this study had comorbidities that could attribute to organ damage accrual prior to SLE diagnosis. SLE generally presents between the ages of 15 and 45 years, with young patients with SLE reporting increased morbidity and mortality rates compared with the general population [6, 32]. It is noteworthy that almost half of the patients in our study were < 45 years of age, indicating a significant burden of SLE is experienced in the young that differs from the general population, which would accumulate organ damage at older ages [33].
Our study reports cumulative renal damage in 13% of patients during 10-year follow-up, which is in agreement with previous reports that suggest that the increase of damage in the renal system is gradual, with incidences of 12–30% between 30 and 35 years post-diagnosis [34]. Cumulative renal damage is associated with low quality of life and high healthcare costs, and therefore a high burden to both the patient and healthcare system.
The group of patients with gonadal organ damage was the only organ system group without greater HCRU and costs in patients with versus without organ damage. The gonadal domain was the least commonly affected domain (17/932 patients [< 2%]) over the follow-up period, and unlike all other organ domains, the proportion of those patients with premature gonadal failure were aged < 45 years (88%). The small numbers of patients with gonadal damage made it hard to observe differences in HCRU and costs in patients with and without gonadal organ damage.
Organ damage progression has been associated with severity of disease activity and corticosteroid dose [35]. Controlling disease activity and avoiding drug-related adverse events from use of standard therapies, e.g., glucocorticoids, therefore, continue to be two of the most important treatment goals in preventing the accumulation of organ damage [36]. Standard therapy for SLE often involves glucocorticoid and immunosuppressant use, which has been associated with both early and late organ damage. In a similar cohort, it has previously been reported that 86.3% of patients were prescribed SLE medications, which included antimalarials, oral glucocorticoids, and/or immunosuppressants [2]. Interestingly, it has been reported that the risk of damage was actually reduced by the antimalarial hydroxychloroquine, suggesting its use may be protective, particularly in renal and neurological systems [37, 38].
Patients with incident organ damage had greater primary (care and prescriptions) and secondary (hospital admission and A&E) HCRU, both before and after their organ damage was diagnosed compared with patients without organ damage, and adjusted mean annualized all-cause healthcare costs were significantly greater for patients with organ damage versus those without. The increased HCRU in patients with organ damage prior to diagnosis was observed most notably in primary care contacts and prescriptions, likely as a result of attempts to manage symptoms, which lead to organ damage and eventual diagnosis. In addition, the organ damage index data may not represent the true onset of organ damage, which may have occurred earlier.
A spike in all-cause healthcare costs was also observed at the time of organ damage diagnosis, which is most likely due to the increased resource use associated with an organ damage diagnosis. These findings are in agreement with results from recent cohort studies in the USA, Europe, and elsewhere, which consistently demonstrate that patients with high disease activity and/or organ damage require more costly care [4, 15,16,17,18, 20]. In one study, modeled 10-year healthcare costs in patients with SLE were up to nine times higher among those with some organ damage at the start of follow-up compared with those without organ damage [21]. This is consistent with our findings, which showed significant differences in regression-adjusted all-cause annual costs per patient with versus without organ damage over the 10-year follow-up. Here, we observed that the healthcare costs for patients with SLE in the UK are substantial, remain high after diagnosis, and rise with the occurrence of organ damage following SLE diagnosis.
Primary care utilization was the leading component of costs during the first year of diagnosis. Although studies in other countries found secondary care, e.g., inpatient stays, to be the largest component of costs associated with SLE within the first 2 years following diagnosis [39], this study found that inpatient stays represented a smaller share of the costs, which might reflect differences in care delivery and costs for inpatient care in the UK versus the USA.
The limitations of this study are common to retrospective observational studies using routinely collected electronic health records data. CPRD GOLD linkage data are only available for 50% of contributing practices in the UK HES and may include missing data, misclassification biases, or inconsistencies in coding within and between practices and over time; however, to reduce potential misclassification, we required linkage with HES and at least 12 months of follow-up for the HCRU cohort. The requirement for the use of verifying information to confirm SLE may have introduced potential bias by underestimating the number of SLE cases, and overestimating patients with more severe disease. Patients with milder disease who did not suffer an exacerbation during the data collection period would not have had the required additional confirmation criteria in their record to be included, resulting in a patient population more likely to have more severe disease. However, only 2% of patients were excluded because of a lack of additional verifying information, indicating that any potential bias may be small. Furthermore, given the requirements to confirm SLE based on these additional confirmation criteria, which included corticosteroid use, it would be challenging to distinguish between organ damage caused by corticosteroid therapy versus SLE disease activity.
There are a number of challenges with using retrospective administrative data for defining organ damage and these limitations should be considered when interpreting the results of this study. Organ damage measures such as the SDI, which requires completion by a physician, complete history and physical examination, chart review, imaging and ophthalmological examination [24] are not routinely captured in real-world databases or administrative and claims data, and it is possible that use of READ and ICD-10 codes to identify organ damage may have overestimated organ damage incidence and led to an underestimation of organ damage costs. The routine capture of validated measures of organ damage in electronic health records and claims data would improve future observational research. Our study design intended to identify incident cases, but the study population may have included prevalent SLE cases without a healthcare encounter or newly insured during the prior year. Future studies including an assessment of disease activity and treatment subgroups and a matched cohort of patients without SLE would add further validation of the extent of damage accrual and subsequent healthcare costs in an SLE cohort and how it varies by severity and treatment type.
One of the strengths of this study is the use of individual patient-level data extracted from a large nationwide general practice records database and linked to hospital and mortality records. The study cohort is representative of the UK general population, and findings are generalizable to patients with SLE treated in UK primary care.
Conclusion
This is the first study to quantify the impact of organ damage on HCRU/costs in patients newly diagnosed with SLE in the UK. Over half of patients are diagnosed with organ damage during the initial years after SLE diagnosis, and patients with organ damage have higher primary and secondary care HCRU and adjusted annualized all-cause healthcare costs than patients without organ damage, both before and after their organ damage is diagnosed. More effective management of SLE may slow disease progression and prevent onset of organ damage, which may translate into improved clinical outcomes and lower healthcare costs.
References
Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78:736–45.
Langham J, Barut V, Samnaliev M, et al. Disease severity, flares and treatment patterns in adults with systemic lupus erythematosus in the UK: a real-world observational retrospective cohort analysis. Rheumatol Adv Pract. 2021;5: rkab061.
Katz P, Nelson WW, Daly RP, Topf L, Connolly-Strong E, Reed ML. Patient-reported lupus flare symptoms are associated with worsened patient outcomes and increased economic burden. J Manag Care Spec Pharm. 2020;26:275–83.
Hammond ER, Desta B, Near AM, Wang X, Jiang M. Frequency, severity and costs of flares increase with disease severity in newly diagnosed systemic lupus erythematosus: a real-world cohort study, United States, 2004–2015. Lupus Sci Med. 2021;8:e000504.
Justiz Vaillant AA, Goyal A, Bansal P, et al. Systemic lupus erythematosus. In: Varacallo M, Aeby T, Mahdy H, editors. StatPearls. Treasure Island: StatPearls Publishing LLC; 2021.
Murimi-Worstell IB, Lin DH, Nab H, et al. Association between organ damage and mortality in systemic lupus erythematosus: a systematic review and meta-analysis. BMJ Open. 2020;10: e031850.
Segura BT, Bernstein BS, McDonnell T, et al. Damage accrual and mortality over long-term follow-up in 300 patients with systemic lupus erythematosus in a multi-ethnic British cohort. Rheumatology (Oxford). 2020;59:698.
Chambers SA, Allen E, Rahman A, Isenberg D. Damage and mortality in a group of British patients with systemic lupus erythematosus followed up for over 10 years. Rheumatology (Oxford). 2009;48:673–5.
Drenkard C, Lim SS. Update on lupus epidemiology: advancing health disparities research through the study of minority populations. Curr Opin Rheumatol. 2019;31:689–96.
Lewis MJ, Jawad AS. The effect of ethnicity and genetic ancestry on the epidemiology, clinical features and outcome of systemic lupus erythematosus. Rheumatology (Oxford). 2016;56:i67–77.
Shi Y, Li M, Liu L, et al. Relationship between disease activity, organ damage and health-related quality of life in patients with systemic lupus erythematosus: a systemic review and meta-analysis. Autoimmun Rev. 2021;20: 102691.
Louthrenoo W, Kasitanon N, Morand E, Kandane-Rathnayake R. Comparison of performance of specific (SLEQOL) and generic (SF36) health-related quality of life questionnaires and their associations with disease status of systemic lupus erythematosus: a longitudinal study. Arthritis Res Ther. 2020;22:8.
Legge A, Doucette S, Hanly JG. Predictors of organ damage progression and effect on health-related quality of life in systemic lupus erythematosus. J Rheumatol. 2016;43:1050–6.
Bertsias G, Karampli E, Sidiropoulos P, et al. Clinical and financial burden of active lupus in Greece: a nationwide study. Lupus. 2016;25:1385–94.
Jiang M, Near AM, Desta B, Wang X, Hammond ER. Disease and economic burden increase with systemic lupus erythematosus severity 1 year before and after diagnosis: a real-world cohort study, United States, 2004–2015. Lupus Sci Med. 2021;8: e000503.
Samnaliev M, Barut V, Weir S, et al. Health-care utilization and costs in adults with systemic lupus erythematosus in the United Kingdom: a real-world observational retrospective cohort analysis. Rheumatol Adv Pract. 2021;5: rkab071.
Schwarting A, Friedel H, Garal-Pantaler E, et al. The burden of systemic lupus erythematosus in Germany: incidence, prevalence, and healthcare resource utilization. Rheumatol Ther. 2021;8:375–93.
Clarke AE, Yazdany J, Kabadi SM, et al. The economic burden of systemic lupus erythematosus in commercially- and Medicaid-insured populations in the United States. Semin Arthritis Rheum. 2020;50:759–68.
Gordon C, Amissah-Arthur MB, Gayed M, et al. The British Society for Rheumatology guideline for the management of systemic lupus erythematosus in adults: executive summary. Rheumatology (Oxford). 2018;57:14–8.
McCormick N, Marra CA, Sadatsafavi M, Aviña-Zubieta JA. Incremental direct medical costs of systemic lupus erythematosus patients in the years preceding diagnosis: a general population-based study. Lupus. 2018;27:1247–58.
Barber MRW, Hanly JG, Su L, et al. Economic evaluation of damage accrual in an international systemic lupus erythematosus inception cohort using a multistate model approach. Arthritis Care Res (Hoboken). 2020;72:1800–8.
Rees F, Doherty M, Grainge M, Davenport G, Lanyon P, Zhang W. The incidence and prevalence of systemic lupus erythematosus in the UK, 1999–2012. Ann Rheum Dis. 2016;75:136–41.
Nightingale AL, Davidson JE, Molta CT, Kan HJ, McHugh NJ. Presentation of SLE in UK primary care using the Clinical Practice Research Datalink. Lupus Sci Med. 2017;4: e000172.
Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363–9.
Gladman DD, Urowitz MB, Goldsmith CH, et al. The reliability of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index in patients with systemic lupus erythematosus. Arthritis Rheum. 1997;40:809–13.
Gladman DD, Goldsmith CH, Urowitz MB, et al. The Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) Damage Index for systemic lupus erythematosus international comparison. 2000. https://pubmed.ncbi.nlm.nih.gov/10685799/. Accessed Feb 10, 2021.
Stoll T, Seifert B, Isenberg DA. SLICC/ACR Damage Index is valid, and renal and pulmonary organ scores are predictors of severe outcome in patients with systemic lupus erythematosus. Br J Rheumatol. 1996;35:248–54.
Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ. 1996;313:275–83.
Onukwugha E, McRae J, Kravetz A, Varga S, Khairnar R, Mullins CD. Cost-of-illness studies: an updated review of current methods. Pharmacoeconomics. 2016;34:43–58.
Hansen RB, Simard JF, Faurschou M, Jacobsen S. Distinct patterns of comorbidity prior to diagnosis of incident systemic lupus erythematosus in the Danish population. J Autoimmun. 2021;123: 102692.
Urowitz MB, Gladman DD, Anderson NM, et al. Cardiovascular events prior to or early after diagnosis of Systemic lupus erythematosus in the Systemic Lupus International Collaborating Clinics cohort. Lupus Sci Med. 2016;3: e000143.
Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol. 2016;12:605–20.
Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–62.
Taraborelli M, Cavazzana I, Martinazzi N, et al. Organ damage accrual and distribution in systemic lupus erythematosus patients followed-up for more than 10 years. Lupus. 2017;26:1197–204.
Urowitz MB, Gladman DD, Ibañez D, et al. Effect of disease activity on organ damage progression in systemic lupus erythematosus: University of Toronto Lupus Clinic Cohort. J Rheumatol. 2021;48:67–73.
van Vollenhoven RF, Mosca M, Bertsias G, et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann Rheum Dis. 2014;73:958–67.
Petri M, Purvey S, Fang H, Magder LS. Predictors of organ damage in systemic lupus erythematosus: the Hopkins Lupus Cohort. Arthritis Rheum. 2012;64:4021–8.
Petri M. Hydroxychloroquine prevents later damage in SLE. Arthritis Rheum. 2001;44:S280.
Garris C, Jhingran P, Bass D, Engel-Nitz NM, Riedel A, Dennis G. Healthcare utilization and cost of systemic lupus erythematosus in a US managed care health plan. J Med Econ. 2013;16:667–77.
Acknowledgements
Funding
Funding for this work, including the journal's Rapid Service Fee was supported by AstraZeneca. The funder of the study had a role in its design, interpretation of the data, and in the writing of the manuscript. The funder had no role in the conduct, collection, or analysis of the data.
Medical Writing, Editorial, and Other Assistance
Writing and editing assistance was provided by Victoria Alikhan, PhD and Rebecca Jones, PhD of JK Associates Inc., part of Fishawack Health. This assistance was funded by AstraZeneca.
Author Contributions
Sarowar Golam, Barbara Naisbett-Groet, Julia Langham, Sue Langham, Danny Gibson, and Heide Stirnadel-Farrant designed the research. Julia Langham, Sue Langham, and Mihail Samnaliev conducted the research. Mihail Samnaliev performed the statistical analysis. All authors contributed to the data interpretation and revised each draft for important intellectual content. All authors read and approved the final manuscript. Heide Stirnadel-Farrant had primary responsibility for the final content and is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Disclosures
Heide Stirnadel-Farrant, Sarowar Golam, Barbara Naisbett-Groet, and Danny Gibson are employees of AstraZeneca. Julia Langham, Sue Langham, and Mihail Samnaliev are consultants and have worked on behalf of AstraZeneca at the time of the study. Mihail Samnaliev is currently an employee of Axtria.
Compliance with Ethics Guidelines
All patient data and linkage using patient identifiers were anonymized. The study was approved by the CPRD Independent Scientific Advisory Committee (ISAC protocol 17_281) on January 28, 2021.
Data Availability
All data relevant to the study are included in the article or Supplementary Material. This study is based in part on data from CPRD obtained under license from the UK Medicines and Healthcare products Regulatory Agency. The data are provided by patients and collected by the National Health Service as part of their care and support. The Office for National Statistics provided the mortality data. The interpretation and conclusions contained in this study are those of the authors alone. The authors do not own these data and hence are not permitted to share the data in the original form.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Stirnadel-Farrant, H.A., Golam, S.M., Naisbett-Groet, B. et al. Healthcare Resource Use and Costs Associated with Organ Damage in Newly Diagnosed Adults with Systemic Lupus Erythematosus in the UK. Rheumatol Ther 10, 1183–1197 (2023). https://doi.org/10.1007/s40744-023-00567-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-023-00567-9