Abstract
Purpose of the Review
Cancer-associated myositis (CAM) is defined as when cancer appears within 3 years of myositis onset. Dermatomyositis and seronegative immune–mediated necrotizing myopathy are the phenotypes mostly related to cancer. In general, treatment principles in myositis patients with and without CAM are similar. However, some aspects of myositis management are particular to CAM, including (a) the need for a multidisciplinary approach and a close relationship with the oncologist, (b) the presence of immunosuppressive and antineoplastic drug interactions, and (c) the role of the long-term immunosuppressive therapy as a risk factor for cancer relapse or development of a second neoplasm. In this review, we will also discuss immunotherapy in patients treated with checkpoint inhibitors as a treatment for their cancer.
Recent Findings
Studies on cancer risk in patients treated with long-term immunosuppressive drugs, in autoimmune diseases such as systemic lupus erythematosus or rheumatoid arthritis, and in solid organ transplant recipients have shed some light on this topic. Immunotherapy, which has been a great advance for the treatment of some types of malignancy, may be also of interest in CAM, given the special relationship between both disorders.
Summary
Management of CAM is a challenge. In this complex scenario, therapeutic decisions must consider both diseases simultaneously.
Similar content being viewed by others
Introduction
Cancer-associated myositis is a term used to describe patients who develop both cancer and inflammatory myopathy within a 3-year period [1]. This specific frame of time is based on epidemiological studies and is the strongest argument for the diagnosis [2,3,4]. It is not always the case, but some patients develop a parallel course between cancer and myositis (i.e., when cancer improves, the myositis disappears, and when cancer relapses, the myositis flares) the so-called “paraneoplastic” myositis [5]. Although any cancer can be associated with myositis, ovarian cancer seems to be one of the most frequent [3].
Of the several myositis phenotypes [6•], dermatomyositis (DM) and seronegative immune–mediated necrotizing myopathy (IMNM) have been most frequently associated with cancer. Alternatively, other types of myositis such as sporadic inclusion body myositis (sIBM) or the antisynthetase syndrome have been rarely linked to cancer. A systematic review published recently on the topic helps to establish the most important risk factors for the development of cancer in patients with myositis [7•]. Besides the above-mentioned phenotypes, and the obvious association with age, the severity of dysphagia, and the positivity for anti-TIF1γ, antibodies were detected relevant risk factors for malignancy.
Managing cancer and myositis in combination is not easy and requires a multidisciplinary approach to find the right balance between myositis and cancer treatment.
Treatment
Diet and lifestyle
There is no specific dietetic advice that is different for people with myositis, other than the general advice that applies to the general population, such as avoiding or quitting smoking, not consuming alcohol, and following an equilibrated Mediterranean diet. Also, contrary to what was originally thought, multiple studies support the beneficial effect of physical activity even in acute forms of myositis [8•]. Regarding environmental exposures, several reports found that UV light may be associated with dermatomyositis [9, 10]. Therefore, patients with myositis, including those with cancer-associated myositis (CAM), should generally avoid exposure to UV light, protect their skin with a high sun-protection factor, and stay active. General vaccination against pneumococcus, influenza, and the recently described agent SARS-CoV2 is strongly recommended in all myositis patients, more even in patients with cancer [11••].
Pharmacological treatment
There are few differences in the pharmacological approach to the treatment of patients with CAM in comparison with those of myositis patients without malignancy. Main pharmacologic groups used to treat CAM, including glucocorticoids, intravenous immunoglobulins, and immunosuppressant antimetabolites such as methotrexate or azathioprine, mycophenolate, calcineurin, antagonists (cyclosporin or tacrolimus), or biological agents (rituximab). Alkylating agents such as cyclophosphamide are exceptionally used in patients with myositis favoring less toxic drugs.
Although refractoriness to therapy has been proposed as a factor suggesting the presence of occult malignancy in patients with myositis [12], this is not our experience, and this has not been identified as a risk factor in the recent systematic review and meta-analysis on 69 articles on CAM [7•].
The most effective therapeutical approach in CAM is not different from non-CAM patients including a combination of prednisone (1 mg/kg/day), calcineurin antagonists (cyclosporine, up to 5 mg/kg/day, or tacrolimus, 0.06 mg/kg/day), and intravenous immunoglobulins (2 g per kg every 4–6 w). Although other immunosuppressive drugs (i.e., mycophenolate mofetil, and others) may be administered instead of calcineurin inhibitors which may present interactions with most antineoplastic agents (see below and in Tables 1 and 2), this is nonetheless our preferred choice due to its efficacy, and we rely on the levels of the drug and frequent arterial pressure measure to avoid toxicities [5]. Interstitial lung disease (ILD) is rare in CAM patients, so specific therapy for ILD is usually not applied. In a recently published cohort from Japan, only 3.6% of patients with CAM had some degree of ILD, and this did not seem to have a significant impact on mortality, which was mostly dependent on the outcomes of the malignancy [13].
In those cases of CAM in whom high-intensity chemotherapy is administered, such as R-CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone-rituximab) in the setting of myositis patients and lymphoproliferative disease, it may be difficult to ascertain whether the myositis improved by resolution of the malignancy or by the immunosuppressive properties of the chemotherapy. This could be a difficulty during the management of these patients, particularly to decide if the immunosuppressive therapy must be maintained afterward. Myositis can rapidly improve when the tumor can be surgically removed. In a retrospective analysis of a large Hungarian myositis cohort, 43 patients were diagnosed with CAM, and those where the tumor could be surgically removed experienced a dramatic improvement of the creatin-kinase values, being difficult to know if this was the consequence of the surgery or to medical therapy [14].
Multidisciplinary approach
Myositis spectrum disorders are considered connective tissue diseases. Although in some phenotypes such as the immune-mediated necrotizing myopathies the main target is the muscle, in dermatomyositis or overlap myositis, several organs may be involved, including the lungs, joints, skin, or even the gut or the heart [6•]. Thus, physicians who usually treat patients with myositis are used to a multidisciplinary approach, requiring the expertise of pulmonologists when interstitial pneumonia is present in patients with the antisynthetase syndrome, or the dermatologist when refractory skin rashes appear in dermatomyositis. The same approach must be used in CAM. Physicians treating myositis need to communicate effectively with the oncologist to improve the outcomes of both diseases. For instance, Taxol/paclitaxel chemotherapy for breast or ovarian cancer may cause weakness due to peripheral neuropathy, which should be differentiated from myositis activity. Muscle magnetic resonance imaging and electromyography can help to differentiate muscle from nerve involvement in these cases. Moreover, capecitabine, a chemotherapy agent which is a prodrug of 5-fluorouracil used for the treatment of metastatic breast cancer, has been reported as a cause of dermatomyositis-like rash [15, 16]. The same clinical dilemma may appear in patients with dermatomyositis associated with a myelodysplastic syndrome or a myeloproliferative disorder (i.e., polycythemia vera) under treatment with hydroxyurea since this drug can cause dermatomyositis-like rash [17].
Lastly, one of the most relevant issues when treating patients with CAM is to identify drug-drug interactions between the immunosuppressive therapy for myositis and the antineoplastic agents which are prescribed for the malignancy. This topic is going to be discussed in the following section.
Immunosuppressive-antineoplastic drug interactions
As it was already mentioned, interactions between cancer and myositis treatments are frequent. Besides changes in the activity of their immunosuppressive and myelosuppressive effects when used together, antineoplastic agents and those used to treat CAM (which occasionally overlap) can present further pharmacokinetic and pharmacodynamic interactions. The most relevant interactions between the most common agents used to treat CAM and the classical and novel antineoplastic therapies can be found in Tables 1 and 2, respectively. For a more detailed description of the mechanism of interaction, severity, and recommended actions, see Supplementary tables S1 and S2 [18,19,20]
Regarding classical antineoplastic agents, some immunosuppressants used to treat CAM such as cyclosporine (a substratum of CYP3A4 but also a P-glycoprotein/ABCB1 and BCRP/ABCG2 inhibitor), leflunomide (CYP1A2 inducer, and CYP2C8 and BCRP/ABCG2 inhibitor), cyclophosphamide (CYP3A4 and CYP2C8 inducer) and aminoquinolines (CYP2D6 inhibitors, and substrates of CYP3A4, CYP3A5, and CYP2C8) can interact with many chemotherapies, such as nitrogen mustard analogs, alkyl sulfonates, topoisomerase 1 (TOP1) inhibitors, and actinomycin (see Table 1). These interactions are caused mainly due to their interference in hepatic metabolic pathways or by concomitant adverse/toxic effects, like the cardiotoxicity of cyclophosphamide or the QT-prolonging effect of aminoquinolines (chloroquine and hydroxychloroquine) and tacrolimus. Due to rhythm alterations, a therapy modification should be considered especially when combining aminoquinolines and pyrimidine analogues or other classical antineoplastic agents, and ALK inhibitors or other protein kinase inhibitors, among other novel antineoplastic therapies. It is worth remarking that concomitant treatment with immunotherapies like the talimogene laherparepvec and BCG vaccine with some antineoplastic drugs might lead to infection by herpes virus simplex (see Supplementary Table S1).
Certain novel antineoplastic agents interact also with calcineurin inhibitors such as cyclosporine and tacrolimus (see Table 2). Again, most of these interactions can be explained because some of the novel antineoplastic agents such as protein kinase inhibitors block the cytochrome P450 or P-glycoprotein metabolism and can increase plasma concentrations of calcineurin inhibitors and their toxicity. On the other hand, although it is a minor interaction that does not preclude their administration, the risk of immunogenic reactions (such as anaphylaxis or serum sickness-type reactions) caused by the unspecific intravascular immunoglobulins may be increased when are used together with monoclonal antibodies and antibody–drug conjugates (see Supplementary Table S2) [18,19,20]
In clinical practice, it is important to be aware of the potential pharmacokinetic interactions between calcineurin inhibitors or aminoquinolines, and some antineoplastic drugs. These agents can also have synergistic immunosuppressive and myelosuppressive effects when used together. Also, besides the role of chloroquine in hepatic metabolic pathways, it may influence in renal excretion of most of classical antineoplastic therapies, ALK inhibitors, and other protein kinase inhibitors; to note, there is an increased risk of muscle toxicity with cladribine, cytarabine, letrozole, methotrexate, and procarbazine.
Immunosuppression, malignancy relapse, and second neoplasm
Immunosuppression may play a role in the development of cancer but not at the initial diagnosis of malignancy associated with myositis. No study has until now definitely linked immunosuppression to cancer-associated myositis [21]. Nevertheless, there are several case reports of post-transplantation lymphoproliferative disorders (PTLD), usually diffuse large B cell lymphomas, in patients with severe immunodepression for refractory myositis, mainly due to the combination of at least two immunosuppressive drugs (i.e., azathioprine plus methotrexate or azathioprine plus mycophenolate mofetil). However, this is an unusual scenario [22].
Also, physicians may consider the risk of cancer progression, relapse of the malignancy, and apparition of a second cancer if severe and long-term immunosuppression is required in patients with CAM. There are no studies addressing this topic in inflammatory myopathy, and although we must consider that inflammatory myopathy has a special relationship with cancer, we may extrapolate data from other diseases such as rheumatoid arthritis or solid organ transplantation [23, 24]. In a review on the cancer risk and recurrence in patients with rheumatoid arthritis who are being treated with biological agents, specifically anti-TNF, or new disease-modifying antirheumatic drugs (DMARD), results from published studies could not detect a higher risk of cancer or recurrence [23]. However, recently, a randomized, open-label study comparing patients with rheumatoid arthritis, who have been treated with TNF inhibitors with those who received tofacitinib a JAK inhibitor drug, found that after 4 years of follow-up, the hazard ratio for cancer was 1.48 in those treated with tofacitinib [25•]. Tofacitinib is being increasingly used in some specific myositis phenotypes such as the anti-MDA5 syndrome or in refractory rashes in dermatomyositis [26, 27].
In patients with myositis, TNF inhibitors are no longer administered given their lack of efficacy [28], and other biologic therapies (IL-6 inhibitors, abatacept), rarely administered in myositis, do not seem to be associated with a higher risk of cancer, recurrence, or even of second neoplasms. Regarding the most used biologic therapy in myositis, which is rituximab, a monoclonal antibody against CD20, there was no association between this drug and the presence of cancer [29, 30]. In other systemic autoimmune diseases, like systemic lupus erythematosus, multidrug therapy using up to three immunosuppressive drugs (cyclosporin, mycophenolate, and glucocorticoids) does not seem to increase the risk of malignancy in the long term [31]. Moreover, similar results were obtained from a meta-analysis that assessed the risk of cancer in patients with rheumatoid arthritis exposed to non-TNF inhibitors biologics or tofacitinib therapy [32].
As has been said previously, patients receiving solid organ transplantation are a model of long-term immunosuppression that may impair the immunosurveillance and increase the risk of cancer. These patients have a 2- to fivefold risk to develop a de novo neoplasm than the general population [33], mainly non-melanoma skin cancer, but also other types of malignancy. Glucocorticoids, which are a cornerstone in the treatment of inflammatory myopathy, are not usually considered to be associated with second neoplasms in immunocompromised patients. Moreover, a systematic review in kidney transplantation patients revealed that after a 5-year follow-up, there were no differences in the occurrence of malignancy between those who received or those who did not require steroid treatment [34]. Azathioprine, an antimetabolite agent that blocks the purine synthesis pathway, that plays a role in the treatment of inflammatory myopathy has been associated with squamous cell carcinoma, with a significantly higher risk than other immunosuppressive drugs, also useful in myositis, such as mycophenolate mofetil or calcineurin inhibitors (cyclosporin or tacrolimus) [35, 36]. Mycophenolate mofetil, which acts as an inhibitor of the de novo guanosine nucleotides synthesis, is a useful agent for the control of some myositis manifestations, mainly skin rashes, ILD, or muscle involvement. A systematic review focused on the cancer risk in solid organ transplant recipients published in 2021 did not find an association between exposure to mycophenolate and an increased risk of cancer; moreover, it may even be associated with a lower risk in comparison with other immunosuppressive agents, such as azathioprine [37••]. Finally, clinicians may consider non-immunosuppressive treatments in patients with CAM, such as intravenous immunoglobulins, or, in severe scenarios, even plasmapheresis.
In summary, clinicians must rely on their own experience and extrapolate from data published in other autoimmune diseases such as systemic lupus erythematosus or rheumatoid arthritis, and from solid organ transplantation patients. Common sense and the wise and cautious use of lower levels of immunosuppression may be helpful for CAM patients, especially in the long-term, in our everyday clinical practice.
Immunotherapy, checkpoint inhibitors, and immune-mediated myopathies
Check-point inhibitors, specifically those blocking the PD1/PD1L pathway, have changed the landscape of cancer therapy, at least in several types of tumors such as small-cell lung cancer and melanoma (SCLC) [38]. Activation of the immune response against the tumor by blocking this pathway has been proved a useful approach in such malignancies. Autoimmune diseases have been excluded from most clinical trials with immunotherapy given the risk of autoimmune disease flaring after the therapy and the possible development of immune-related adverse events (irAE) [39,40,41,42].
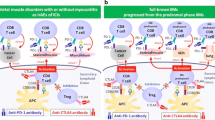
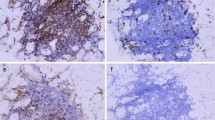
Although several authors have reported dermatomyositis patients with cancer in whom the disease flares after the therapy with checkpoint inhibitor [43,44,45,46], this is not always the rule, and studies addressed to a better definition of those patients in whom immunotherapy will be suitable are being conducted. But this is only one side of the coin, the other side refers to those patients with cancer who develop an immune-mediated myopathy soon after the onset of immunotherapy. Sometimes, it may be difficult to differentiate if it is a paraneoplastic myositis or an immune-related adverse event [47]. Nevertheless, the clinical context and the muscle biopsy may help to differentiate between both scenarios. Paraneoplastic dermatomyositis is usually accompanied by characteristic skin lesions (i.e., Heliotrope rash or Gottron papules) and positivity to specific myositis antibodies (i.e., mainly anti-TIF1γ, but also anti-SAE or anti-NXP2), and the muscle biopsy often shows perifascicular atrophy or perimysial lymphocyte infiltrates [6•]. Alternatively, checkpoint inhibitor-induced myositis patients have characteristic clinicopathological phenotype combining limb-girdle, axial, and oculomotor weakness with a unique pattern of pseudogranulomatous necrotic infiltrates of macrophages and T cells. Patients with checkpoint inhibitor–induced myositis may develop myocarditis [48, 49•]. Differentiating seronegative IMNM of paraneoplastic origin from IMNM induced by checkpoint inhibitors is currently challenging but clinically relevant. A second shot of immunotherapy should be carefully evaluated in these cases, considering the risk/benefit ratio.
I would like to finish this section, sharing a case report of a patient with CAM who was treated with immunotherapy and partially responded to the therapy.
Clinical vignette
A 66-year-old male was recently diagnosed with dermatomyositis. He complained of severe proximal muscle weakness that prevented him to stand up from a chair or helping in the kitchen. Gottron papules on the knuckles of both hands and a slight heliotrope rash were also present. A muscle biopsy revealed perifascicular atrophy and perimysial lymphocytic infiltrates. The autoimmunity profile disclosed a positivity to anti-NXP2 antibodies and high values of creatinine kinase (1721 IU/l, NV < 192 IU/l). PET/CT performed for occult cancer screening revealed a solid nodule at the inferior left lung lobe with a high standardized uptake value (SUV max = 16) and hypermetabolic mediastinal adenopathies, which suggested malignancy. A left adrenal gland also had a high SUV. A biopsy of a satellite lymphadenopathy was compatible with a small-cell lung cancer. An adrenal MRI confirmed a metastatic lesion, and the cranial CT showed multiple brain metastases.
While the cancer diagnosis work-up was ongoing, the patient received triple therapy with tacrolimus 2 mg/12 h, intravenous immunoglobulins (0.4 g/kg/day × 5 days), and prednisone (1 mg/kg/day) with a moderate improvement at 1 month. While therapy for myositis was instituted, chemotherapy based on carboplatin and etoposide was started with partial response after 4 cycles; then, holocraneal radiotherapy was administrated (total dose 9 Gy). Three months after starting monthly IVIGs while he was receiving a low dose of prednisone, the dermatomyositis was controlled, and the patient was able to come walking to our outpatient clinic, take a shower, and help in the kitchen. Nevertheless, the malignancy outcome was not satisfactory, and multiple brain metastases persisted. Despite the co-occurrence of an autoimmune disease, and after the sponsor authorization, a clinical trial with an anti-PD1 as maintenance therapy was offered to the patient. Pros and cons of immunotherapy were discussed with the patient and his family including the possibility of having a dermatomyositis flair and other autoimmune events. Eventually, the patient accepted, and he was enrolled in the clinical trial.
After evaluating the pros and cons, the oncologist, the doctor in charge of myositis, the patient, and his family decided to start this immunotherapy. The patient remained under immunotherapy treatment for 2 years, with partial response as best overall response. After that, disease progression was confirmed, and he started topotecan, and unfortunately, he passed away due to tumor progression. In the end, there was discrete evidence of clinical activity of his dermatomyositis.
Several issues can be drawn from this clinical vignette. The first issue was that the clinical course of cancer and dermatomyositis does not always run in parallel, and that dermatomyositis, even in patients with CAM, may respond well to the conventional therapy combining, in severe cases, prednisone, IVIGs, and, in this case, tacrolimus. The second issue was that immunotherapy may be useful, at least in some cases of myositis, as is exemplified in the present case. Different factors, like the type of tumor, its level of differentiation, and the density of lymphocytes infiltrating the tumor (“hot tumors”), may help in the future to identify ideal candidates to receive immunotherapy and prevent irAE. Given the special relationship between cancer and dermatomyositis, often paraneoplastic, it would not be surprising that the approach regarding immunotherapy ends up being different than in patients with other autoimmune diseases.
Conclusions
Management of cancer-associated myositis, a condition that may be diagnosed when cancer and myositis appear within a period of 3 years, may be a challenge. Working together with the oncologists is mandatory to achieve the best possible outcome in these patients. Pharmacologic interactions between immunosuppressants and antineoplastic agents must be addressed also. Clinicians must consider the risk of long-term immunosuppression in those patients in terms of cancer relapse or development of a second malignancy. Given the absence of data in idiopathic inflammatory myopathies, lessons can be drawn from other systemic diseases such as systemic lupus erythematosus or rheumatoid arthritis treated with severe and long-term immunosuppression or from solid transplantation recipients. The role of immunotherapy as a useful approach to cancer and the risk of flare that these patients may develop are under discussion.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Troyanov Y, Targoff IN, Tremblay JL, Goulet JR, Raymond Y, Senécal JL. Novel classification of idiopathic inflammatory myopathies based on overlap syndrome features and autoantibodies: analysis of 100 French Canadian patients. Medicine (Baltimore). 2005;84:231–49.
Sigurgeirsson B, Lindelof B, Edhag O, Allander E. Risk of cancer in patients with dermatomyositis or polymyositis. A population-based study. N Engl J Med. 1992;326:363–7.
Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96–100.
Buchbinder R, Forbes A, Hall S, Dennett X, Giles G. Incidence of malignant disease in biopsy-proven inflammatory myopathy. A population-based cohort study. Ann Intern Med. 2001;134:1087–95.
Selva-O’Callaghan A, Trallero-Araguás E. Myositis and cancer. In: Graus F, Editor-in-Chief. MedLink Neurology. San Diego: MedLink LLC. Available at www.medlink.com. Updated: [8.31.2021].
Selva-O’Callaghan A, Pinal-Fernández I, Trallero-Araguás E, Milisenda JC, Grau-Junyent JM, Mammen AL. Classification and management of adult inflammatory myopathies. Lancet Neurol. 2018;17:816–28. A comprehensive review on the classification and management of inflammatory myopathies that include the most accepted clinic-immunological phenotypes.
Oldroyd AGS, Allard AB, Callen JP, et al. A systematic review and meta-analysis to inform cancer screening guidelines in idiopathic inflammatory myopathies. Rheumatology (Oxford). 2021;60:2615–28. In this systematic review and meta-analysis of 69 studies, main cancer risk factors in patients with idiopathic inflammatory myopathies are described.
Alexanderson H, Boström C. Exercise therapy in patients with idiopathic inflammatory myopathies and systemic lupus erythematosus — a systematic literature review. Best Pract Res Clin Rheumatol. 2020;34:101547. An excellent review focused on the physical therapy and its benefits in patients with myositis.
Okada S, Weatherhead E, Targoff IN, Wesley R, Miller FW. Global surface ultraviolet radiation intensity may modulate the clinical and immunologic expression of autoimmune muscle disease. Arthritis Rheum. 2003;48:2285–93.
Love LA, Weinberg CR, McConnaughey DR, et al. Ultraviolet radiation intensity predicts the relative distribution of dermatomyositis and anti-Mi-2 autoantibodies in women. Arthritis Rheum. 2009;60:2499–504.
Shinjo SK, de Souza FHC, Borges IBP, et al. Systemic autoimmune myopathies: a prospective phase 4 controlled trial of an inactivated virus vaccine against SARS-CoV-2. Rheumatology (Oxford). 2021;19:keab773. https://doi.org/10.1093/rheumatology/keab773. This prospective controlled study demonstrates that Sinovac-Coronavac is safe for patients with myositis although the presence of neutralizing antibodies was reduced in comparison with a control group.
Moghadam-Kia S, Oddis CV, Ascherman DP, Aggarwal R. Risk factors and cancer screening in myositis. Rheum Dis Clin North Am. 2020;46:565–76.
Kaneko Y, Ninawa T, Taniguchi Y, et al. JAMI investigators. Clinical characteristics of cancer-associated myositis complicated by interstitial lung disease: a large-scale multicentre cohort study. Rheumatology (Oxford). 2020;59:112–9.
András C, Bodoki L, Nagy-Vincze M, Griger Z, Csiki E, Dankó K. Retrospective analysis of cancer-associated myositis patients over the past 3 decades in a Hungarian myositis cohort. Pathol Oncol Res. 2020;26:1749–55.
Saraswat N, Verma R, Neema S, Kumar S. A case of capecitabine-induced dermatomyositis. Indian J Pharmacol. 2018;50:350–3.
Chen FW, Zhou X, Egbert BM, Swetter SM, Sarin KY. Dermatomyositis associated with capecitabine in the setting of malignancy. J Am Acad Dermatol. 2014;70:e47–8.
Dacey MJ, Callen JP. Hydroxyurea-induced dermatomyositis-like eruption. J Am Acad Dermatol. 2003;48:439–41.
European Medicines Agency (EMA). Medicinal products for human use. Summary of Product Characteristics, 2022. Available at: https://www.ema.europa.eu/en/human-medicines-regulatory-information. Last access: 25th January 2022.
Food and Drug Administration (FDA). Drugs. Summary of Product Characteristics and Safety Information, 2022. Available at: https://www.fda.gov/Drugs. Last access: 25th January 2022.
Wishart DS, Knox C, Guo AC, et al. Drug bank: a knowledge base for drugs, drug actions and drug targets. Nucleic Acids Res. 2008;36(Database issue):D901-6.
Tiniakou E, Mammen AL. Idiopathic inflammatory myopathies and malignancy: a comprehensive review. Clin Rev Allergy Immunol. 2017;52:20–33.
Selva-O’Callaghan A, Palacios A, Solans-Laque R, Labirua A, Salcedo-Allende T, Vilardell-Tarrés M. Epstein-Barr virus-associated lymphoma in patients with dermatomyositis. Be aware of double immunosuppression. Rheumatology (Oxford). 2009;48:1462–3.
Singh N, Li CI. Impact of rheumatoid arthritis and biologic and targeted synthetic disease modifying antirheumatic agents on cancer risk and recurrence. Curr Opin Rheumatol. 2021;33:292–9.
Cangemi M, Montico B, Faè DA, Steffan A, Dolcetti R. Dissecting the multiplicity of immune effects of immunosuppressive drugs to better predict the risk of de novo malignancies in solid organ transplant patients. Front Oncol. 2019;9:160.
Ytterberg SR, Bhatt DL, Mikuls TR, et al. ORAL Surveillance investigators Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316–26. A higher risk of cancer was detected in patients with rheumatoid arthritis treated with tofacitinib in comparison with those treated with TNF inhibitors after a period of 4 years follow-up.
Chen Z, Wang X, Ye S. Tofacitinib in amyopathic dermatomyositis-associated interstitial lung disease. N Engl J Med. 2019;381:291–3.
Paik JJ, Casciola-Rosen L, Shin JY, et al. Study of tofacitinib in refractory dermatomyositis: an open-label pilot study of ten patients. Arthritis Rheumatol. 2021;73:858–65.
Dastmalchi M, Grundtman C, Alexanderson H, et al. A high incidence of disease flares in an open pilot study of infliximab in patients with refractory inflammatory myopathies. Ann Rheum Dis. 2008;67:1670–7.
Wadstrom H, Frisell T, Askling J, Anti-rheumatic therapy in Sweden study G. malignant neoplasms in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors, tocilizumab, abatacept, or rituximab in clinical practice: a nationwide cohort study from Sweden. JAMA Intern Med. 2017;177:1605–12.
Emery P, Furst DE, Kirchner P, et al. Risk of malignancies in patients with rheumatoid arthritis treated with rituximab: analyses of global postmarketing safety data and long-term clinical trial data. Rheumatol Ther. 2020;7:121–31.
Shin JI, Li H, Park S, et al. Induction and maintenance treatment of Lupus nephritis: a comprehensive review of meta-analyses. J Clin Med. 2022;11:343.
Xie W, Yang X, Huang H, Gao D, Ji L, Zhang Z. Risk of malignancy with non-TNFi biologic or tofacitinib therapy in rheumatoid arthritis: a meta-analysis of observational studies. Semin Arthritis Rheum. 2020;50:930–7.
Hall EC, Pfeiffer RM, Segev DL, Engels EA. Cumulative incidence of cancer after solid organ transplantation. Cancer. 2013;119:2300–8.
Haller MC, Royuela A, Nagler EV, Pascual J, Webster AC. Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database Syst Rev. 2016;22:CD005632.
Cho HG, Kuo KY, Xiao K, et al. Azathioprine and risk of multiple keratinocyte cancers. J Am Acad Dermatol. 2018;78:27–8.
Jiyad Z, Olsen CM, Burke MT, Isbel NM, Green AC. Azathioprine and risk of skin cancer in organ transplant recipients: systematic review and meta-analysis. Am J Transplant. 2016;16:3490–503.
Hirunsatitpron P, Hanprasertpong N, Noppakun K, Pruksakorn D, Teekachunhatean S, Koonrungsesomboon N. Mycophenolic acid and cancer risk in solid organ transplant recipients: systematic review and meta-analysis. Br J Clin Pharmacol. 2021;8. https://doi.org/10.1111/bcp.14979. Data retrieved from this systematic review and meta-analysis help to establish the true risk of cancer in patients receiving therapy with mycophenolate mofetil. This therapy was not associated with an increased risk of cancer.
Le Saux O, Lounici Y, Wajda P, et al. Neoadjuvant immune checkpoint inhibitors in cancer, current state of the art. Crit Rev Oncol Hematol. 2021;157:103172.
Florou V, Puri S, Garrido-Laguna I, Wilky BA. Considerations for immunotherapy in patients with cancer and comorbid immune dysfunction. Ann Transl Med. 2021;9:1035.
Abdel-Wahab N, Shah M, López-Olivo MA, et al. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease. Ann Intern Med. 2018;169:133.
Tison A, Quéré G, Misery L, et al. Safety and efficacy of immune checkpoint inhibitors in patients with cancer and preexisting autoimmune disease: a nationwide, multicenter cohort study. Arthritis Rheumatol. 2019;71:2100–11.
Kaur A, Doberstein T, Amberker RR, et al. Immune-related adverse events in cancer patients treated with immune checkpoint inhibitors. Medicine (Baltimore). 2019;98:e17348.
Takatsuki K, Yanagihara T, Egashira A, et al. A rare case of pembrolizumab-induced dermatomyositis in a patient with cancer of unknown primary origin. Am J Case Rep. 2021;22:e930286.
Thomas R, Patel H, Scott J. Dermatomyositis flare with immune checkpoint inhibitor therapy for melanoma. Cureus. 2021;13:e14387.
Messer A, Drozd B, Glitza IC, Lu H, Patel AB. Dermatomyositis associated with nivolumab therapy for melanoma: a case report and review of the literature. Dermatol Online J. 2020;26:13030/qt4c21b068.
Kosche C, Stout M, Sosman J, Lukas RV, Choi JN. Dermatomyositis in a patient undergoing nivolumab therapy for metastatic melanoma: a case report and review of the literature. Melanoma Res. 2020;30:313–6.
Shibata C, Kato J, Toda N, et al. Paraneoplastic dermatomyositis appearing after nivolumab therapy for gastric cancer: a case report. J Med Case Rep. 2019;13:168.
Selva-O’Callaghan A, Trallero-Araguás E, Milisenda JC, Grau-Junyent JM. Differential diagnosis of necrotizing myopathy. Curr Opin Rheumatol. 2021;33:544–53.
Matas-García A, Milisenda JC, Selva-O'Callaghan A, et al. Emerging PD-1 and PD-1L inhibitors-associated myopathy with a characteristic histopathological pattern. Autoimmun Rev. 2020; 19:102455. A characteristic clinical and pathological picture was depicted in the analysis of patients with checkpoint inhibitors associated myopathy.
Funding
This study was funded by the Instituto de Salud Carlos III Grant PI18/01609 co-financed by the European Regional Development Fund (ERDF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Albert Selva-O’Callaghan declares that he has no conflict of interest. Ernesto Trallero-Araguás declares that he has no conflict of interest. Javier Ros declares that he has no conflict of interest. Albert Gil-Vila declares that he has no conflict of interest. Julia Lostes declares that she has no conflict of interest. Antonia Agustí declares that she has no conflict of interest. Judit Riera-Arnau declares that she has no conflict of interest. Marcelo Alvarado-Cárdenas declares that he has no conflict of interest. Iago Pinal-Fernandez declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Special thanks to Dr. Ivan Castellvi for reviewing
This article is part of the Topical Collection on Other CTD: Inflammatory Myopathies and Sjogren’s
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Selva-O’Callaghan, A., Trallero-Araguás, E., Ros, J. et al. Management of Cancer-Associated Myositis. Curr Treat Options in Rheum 8, 91–104 (2022). https://doi.org/10.1007/s40674-022-00197-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40674-022-00197-2