Abstract
Background
This study compared the effectiveness and tolerability of alpha-tocopherol acetate nasal spray (ATANS) to those of beclomethasone nasal spray (BNS) and loratadine (LT) for the treatment of pollen-induced allergic rhinitis.
Methods
This active-controlled, patient-preference, observational trial lasted 7 days. Tested variables were nasal symptoms, endoscopic examination, additional medications, general effectiveness, safety, and tolerability.
Results
Of 116 patients, 63 patients decided to use ATANS, 32 BNS and 21 LT. During the treatment, the mean daily rhinitis symptom score decreased significantly in all groups. The 7‑day mean score in the ATANS group (0.98) was comparable to that of the BNS group (0.92) and significantly higher than that of the LT group (0.70, P < 0.05). After treatment, the endoscopic score was significantly lower in all groups; the reductions were significantly greater in the BNS and LT groups than in the ATANS group (BNS vs. ATANS: P < 0.01; LT vs. ATANS: P < 0.05). Additional medications were not used by 60% (ATANS), 47% (BNS) and 95% (LT) of patients; the difference between ATANS and LT was significant (P < 0.01). Treatment was assessed as good or very good by 56% (ATANS), 69% (BNS) and 86% (LT) of patients; the differences between ATANS and BNS (P < 0.05) as well as between ATANS and LT (P < 0.01) were significant. Most patients (96%) did not experience any adverse events. Pollen intensity was comparable between groups and decreased marginally on day 7.
Conclusion
ATANS can be considered an effective symptomatic treatment for patients with allergic rhinitis who wish to avoid side effects of antihistamines and corticosteroids.
Trial registration
German “Register Klinischer Studien” (Reference number: DRKS00009338)
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
Allergic rhinitis (AR) is a common disease that currently affects 10 to 30% of the worldwide population and is still increasing [1]. The Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines have proposed a stepwise approach for the pharmacological treatment of AR that is commonly based on oral/topical antihistamines and corticosteroid nasal spray [2]. However, like any other drug, antihistamines and intranasal corticosteroids are associated with side effects, some of which might be headache, fatigue, somnolence, and local irritations [3, 4]. More concerns have been raised about the use of these drugs in children: intranasal corticosteroids can reduce bone density and growth velocity; all first-generation and some second-generation antihistamines can impair children’s cognitive function and school performance [5,6,7,8,9,10]. Therefore, children or their care-givers as well as sensitive patients, pregnant women or health-conscious patients might prefer alternative, non-pharmacological therapies.
The pathological mechanisms underlying AR involve IgE-mediated inflammations of the nasal mucosa [11]. Evidence from an animal study showed that vitamin E supplementation reduced the IgE-mediated immune response [12]. In a study conducted in 2633 randomly enrolled adults, those with a higher intake of dietary vitamin E had lower IgE concentrations and a lower frequency of allergen sensitisation [13]. Another study showed that daily use of vitamin E supplements during the pollen season significantly reduced self-reported nasal symptoms in patients with seasonal AR when compared to placebo [14].
Alpha-tocopherol acetate (vitamin E acetate) nasal spray is a vitamin E-based oil which forms a protective film on the nasal mucosa, helps to restore normal hydration and possibly to alleviate the symptoms of AR. In a previous study, adjunctive treatment with alpha-tocopherol acetate nasal spray in addition to guideline-recommended beclomethasone nasal spray and/or antihistamines reduced AR symptoms such as nasal obstruction, rhinorrhoea, and sneezing within minutes after application. As a result, 20 out of 36 patients reduced their intake of medications, and 12 out of 36 patients stopped using medications completely [15]. The application of alpha-tocopherol acetate nasal spray after endoscopic sinus surgery in patients with chronic rhinosinusitis accelerated nasal mucosa healing and prevented the recurrence of complications [16].
This study investigated alpha-tocopherol acetate nasal spray as a comprehensive treatment alternative to guideline-recommended medications for patients with birch, grass, or rye pollen-induced AR. The therapeutic effects and tolerability of the investigational product were compared to those of beclomethasone nasal spray (glucocorticoid nasal spray) and loratadine tablets (antihistamines).
Methods
Study design
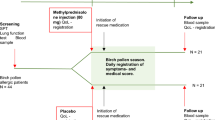
This was a prospective, active-controlled, patient-preference observational study. It was carried out in accordance with the German Medicinal Products Act (Arzneimittelgesetz, AMG), Section 4, subsection 23, sentences 2 and 3, as well as Section 67 subsection 6, and with the German Medical Devices Act (Medizinproduktegesetz, MPG), Section 23b. It was approved by the competent ethics committee of the University Hospital Cologne, Germany, and registered under the reference number 15-092. This study was also registered with the German “Register Klinischer Studien” (Reference number: DRKS00009338). It aimed to observe patients during routine medical practice. Thus, randomisation and placebo control as in clinical trials are not possible according to AMG, Section 4, subsection 23, sentence 1. Instead, patients were free to choose their treatment option before enrolment. In this observational study, we investigated and compared the non-pharmacological therapy (alpha-tocopherol acetate nasal spray, ATANS), glucocorticoid nasal spray (beclomethasone nasal spray, BNS) and systemic antihistamine (loratadine tablets, LT).
We planned to enrol 120 patients aged 18 years or older with pollen-induced AR during the birch and grass pollen season between April and July 2015. Ten to 15 ear, nose and throat trial sites in Germany were to participate in this study. All patients signed an informed consent form on data use before enrolment. The observation lasted 7 days and included an initial visit at the beginning and a final visit at the end of this period.
Study medication
Study medication was administered according to the instructions for use. Alpha-tocopherol acetate (vitamin E) nasal spray is a vitamin E-based oil which forms a protective barrier on the nasal mucosa helping to restore normal hydration (Filme Nasale®, PANIN S.R.L., Rovigo, Italy; CE-marked medical device). It is recommended to pump two puffs into each nostril at least twice a day and up to six times a day as needed. BNS (50 µg/puff) contains the corticosteroid beclometasone dipropionate with anti-inflammatory and anti-allergic properties (ratioAllerg® Heuschnupfenspray, ratiopharm GmbH, Ulm, Germany). The package insert recommends two puffs into each nostril in the morning and evening. LT (Loratadin-ratiopharm®, ratiopharm GmbH, Ulm, Germany) contains 10 mg of the antihistamine loratadine and should be taken once a day.
Clinical assessments
At visit 1 (V1), patients’ demographic data and allergic anamneses were recorded. During 7 days of observation, the patients provided diary data with regard to the use of study medication (nasal sprays and tablets) and rhinitis symptoms including sneezing, rhinorrhoea, nasal pruritus and nasal congestion on a scale from 0 to 3 (0 = none, 1 = mild, 2 = moderate and 3 = severe symptoms) for the assessment of effectiveness [17]. The effectiveness of the treatment was also evaluated by endoscopic examination of the nasal cavity at V1 and V2, and the assessment of oedema, secretion and redness of the nasal mucosa using a 3-point scale (0 = no, 1 = mild/clear or fluid secretion and 2 = severe/thick or mucous secretion). Additionally, at V1 or V2, allergic symptoms and the intake of symptomatic medication in the 2015 pollen season were documented before and during the 7‑day observation period. The intensity of symptoms was evaluated using a 4-point scale (0 = none, 1 = mild, 2 = moderate and 3 = severe symptoms) [17]. The frequency of symptoms was assessed on the following 4‑point scale: 0 = seldom, 1 = <4 weeks’/days’* use, 2 = ≥4 weeks’/days* use and 3 = constant (*days with respect to the 7‑day study period). After the 7‑day observation period, the patients were asked to evaluate the effectiveness of the respective treatment as well as its tolerability on a scale from 1 to 4 (1 = very good, 2 = good, 3 = moderate and 4 = poor). In order to compare the patients’ sensory perception of the sprays, patients using nasal spray filled in questionnaires directly after application and two minutes thereafter. In total, 14 questions concerning sensory parameters were answered by marking a visual analogue scale (0 = poor evaluation, 100 = good evaluation), yielding the Nasal Spray Sensory Scale score [18]. At V2, adverse events (AEs) during the 7‑day observation period were recorded for safety evaluation.
Pollen data
Pollen data were acquired from the German Weather Service, Medicine Meteorology (Deutscher Wetterdienst, Medizin-Meteorology, http://www.dwd.de). The pollen intensity (0 = no pollen, 1 = mild pollen intensity, 2 = moderate pollen intensity, 3 = high pollen intensity) was defined for each patient according to the nearest pollen count station in the region she/he lived in during the 7‑day observational period.
Statistics
The statistical analysis program IBM SPSS for Windows (version 23, IBM Corp., Armonk, NY, USA) was used for data entry and analysis. Demographic data and baseline characteristics as well as effectiveness and tolerability variables were analysed using descriptive statistics for each of the three treatment groups. Continuous data were described by the number of valid or missing values as well as by the mean, median, standard deviation, minimum and maximum. Categorical data were expressed as absolute or percentage frequencies. With regard to continuous and categorical data, the groups were compared using the nonparametric Mann–Whitney U test to assess baseline comparability and differences in effectiveness or tolerability. Results were considered statistically significant if P < 0.05. The last value carried forward option was applied with regard to missing diary data on rhinitis symptoms. A minimum of two entries for each parameter at the beginning of the observation period was necessary for the application of this biometric technique and in order to gain a sum score for each day.
Results
Study population
Ten study centres across Germany participated in this non-interventional study (NIS) and enrolled 118 patients. Two patients did not fulfil the criteria of having birch, grass or rye pollen-induced AR. Thus, the data of 116 patients were analysed. Of these, 63 patients decided to use ATANS, 32 BNS and 21 LT (Table 1; Fig. 1). The first V1 was on 27 April 2015 and the last V2 on 12 August 2015. Two patients in the ATANS group did not complete treatment. Nevertheless, one of those patients delivered diary data for 3 days and attended V2. In summary, 114 patients (98.3%) completed the study and 112 patients (96.6%) delivered completed patient diaries.
The age of study patients ranged from 18 to 76 years, with the mean being 39.1 years. The mean age was similar across all groups (39.1 years in the ATANS group, 39.0 years in the BNS group and 39.2 years in the LT group). The percentage of female patients was higher in all treatment groups (61.0% in the ATANS group, 54.8% in the BNS group and 76.2% in the LT group). The majority of patients in all groups had AR induced by birch pollen alone or by birch plus grass and/or rye pollen (61.9% in the ATANS group, 62.5% in the BNS group and 52.4% in the LT group; Table 1).
Pollen data
The pollen intensity was mild to moderate during the 7‑day observational period and was slightly stronger (non-significant) in the ATANS and BNS groups than in the LT group (Online Resource 1 A, B). It remained almost unchanged in the ATANS group and decreased only marginally in the BNS and LT groups towards the end of the observational period (Online Resource 1 B). The decrease was attributable to birch pollen, while grass pollen remained relatively consistent; grass pollen was more intense than birch pollen, while there was no rye pollen in areas where patients lived in during the study (Online Resource 1 C–E).
Use of study medication
Patients recorded their use of study medication during the 7‑day observation period on a daily basis. ATANS was applied one to three times daily (mean 2.41 ± 0.602, median 2.57), although up to six times daily were allowed according to the instructions for use. BNS (mean 1.95 ± 0.603, median 2.00) and LT (mean 1.00 ± 0.000, median 1.00) were used as recommended by the manufacturers.
Effectiveness of the study medication
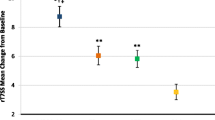
Patients’ diary data regarding the rhinitis symptoms of sneezing, rhinorrhoea, nasal pruritus, and nasal congestion showed a decrease in the intensity of symptoms in all treatment groups. The score decreased from 1.16 ± 0.736 to 0.85 ± 0.613 in the ATANS group, from 1.12 ± 0.743 to 0.85 ± 0.735 in the BNS group and from 1.08 ± 0.757 to 0.68 ± 0.597 in the LT group (27, 24 and 37% reduction, respectively; Fig. 2). In the ATANS group, the first statistically significant reduction in the rhinitis symptom score compared to day 1 was observed on day 6 (P < 0.05). In contrast, statistical significance in the BNS group was reached for the first time on day 4 (P < 0.05) and in the LT group on day 3 (P < 0.01). The mean rhinitis symptom score calculated for the 7‑day observation period was lower in the LT group (0.70) than in the ATANS group (0.98) and showed a statistical significance (P < 0.05). However, between the ATANS and the BNS groups, the difference in the mean rhinitis score over 7 days was not significant (0.98 versus 0.92).
Daily mean rhinitis symptom score. This figure shows the course of the daily mean rhinitis symptom score for the three treatment groups over the study duration of 7 days. Rhinitis symptoms (sneezing, rhinorrhoea, nasal pruritus and nasal congestion) were evaluated on a scale from 0 to 3 (0 = none, 1 = mild, 2 = moderate and 3 = severe symptoms) by the patients in the patient diaries on a daily basis. ATANS alpha-tocopherol acetate nasal spray, BNS beclomethasone nasal spray, LT loratadine tablets
Endoscopic examinations of the nasal cavity at V1 and V2, which included the assessments of oedema, secretion and redness of the nasal mucosa, yielded sum scores that decreased in each treatment group. The differences between V1 and V2 (0.85 in the ATANS group, 2.04 in the BNS group, and 1.90 in the LT group) were statistically significant in each treatment group (P < 0.001 for the ATANS and BNS groups, P < 0.01 for the LT group). The differences in this score were significantly greater in the BNS and LT groups than in the ATANS group (P < 0.01 and P < 0.05, respectively).
The rhinitis score comparing the intensity and the frequency of rhinitis symptoms before and during the treatment decreased significantly in each study group (0.90 in the ATANS group, 1.44 in the BNS group and 1.65 in the LT group). The decrease was significantly greater in the LT group than in the ATANS group (P < 0.05).
At V1 and V2, patients were asked about their use of symptomatic medication. Before the 7‑day observation period, 34 to 44% of patients did not use any symptomatic medication. During treatment with the study medication, 60% of patients in the ATANS group, 47% of patients in the BNS group and 95% of patients in the LT group did not use any additional symptomatic medication. The difference in the percentage of patients not using additional medication during the study period was statistically significant when comparing the ATANS and LT groups (P < 0.01; Fig. 3).
At the end of the observation period, the patients evaluated the effectiveness of their treatment. The majority of patients in each group (56% of ATANS patients, 69% of BNS patients and 86% of LT patients) assessed their treatment as good and very good. The differences between the ATANS group and the BNS group and between the ATANS group and the LT group were statistically significant (P < 0.05 and P < 0.01, respectively; Fig. 4).
Tolerability and safety of the study treatment
The tolerability of the respective treatment was assessed as good or very good by most of the patients in each treatment group (60% of ATANS patients, 88% of BNS patients and 90% of LT patients). The differences between the ATANS group and the BNS group and between the ATANS group and the LT group were statistically significant (P < 0.01 in both cases).
The mean sensory perception scores assessed by the patients in the ATANS and the BNS groups ranged from 75 to 83, testifying a rather comfortable feeling. There were no significant differences in this score between the ATANS and BNS groups directly after application or 2 min thereafter.
Five AEs were reported in the ATANS group, which were related or possibly related to the study medication. AEs such as oily taste, throat discomfort or sore throat were mild or moderate. Two severe AEs (rhinitis, sneezing and pharyngeal irritation) were documented, and the respective patients discontinued the study medication. Ninety-six percent of patients had no AEs. No serious AEs or fatalities occurred in this NIS.
Discussion
This study was conducted as an observational (non-interventional) study and not as a randomised clinical trial according to the German Medicinal Products Act, Section 4, subsection 23, sentence 1. Observational studies provide valuable insights on how a treatment is used in routine clinical practice. The patient population in an observational study represents the real-life population and is not selected by restrictive inclusion criteria, unlike those in many randomised, placebo-controlled trials. Observational studies include all patients who seek treatment and who fit in the study design. Results from an observational study reflect the effectiveness, safety and tolerability of the treatment under real-life conditions. Such results are influenced by real-life factors such as the patients’ preference, knowledge and understanding of the treatment, as well as by the concomitant medication used at the patients’ discretion [19]. This study design is suitable for products that have been authorised for use or for medical devices with a CE marking, and it was applied in this investigation. Nevertheless, the weaknesses of this study were the lack of placebo control and randomisation. To overcome these problems, we used pollen data to ensure comparable pollen exposure and validate the comparisons between three groups. We chose an observational period of 7 days instead of 14 days as recommended by the Food and Drug Administration (FDA) and the European Agency for the Evaluation of Medicinal Products (EMEA) [20] to ensure a high treatment compliance and completeness of the patient diaries. As a result, 98.3% of patients completed our study and 96.6% of patients delivered completed diaries. Furthermore, according to the guideline of the German society for allergology and clinical immunology [21], the protracted start of nasal corticosteroid effects is within hours to 1 day of application. In clinical trials, the first significant reduction in rhinitis symptom score is usually observed within the first few days of treatment, which was also confirmed in our study (BNS: day 4, P < 0.05; LT: day 3, P < 0.01; ATANS: day 6, P < 0.05). Thus, the 7‑day observational period appeared to be sufficient to show the effectiveness of all three treatments.
This study compared, for the first time, the effectiveness and tolerability of ATANS with those of two guideline-recommended therapies—BNS and LT. Even though there were significantly more BNS patients who rated the treatment as good or very good than ATANS patients who did so (69% vs. 56%, P < 0.05), two other factors—the rhinitis symptom score and the use of additional medication—demonstrated comparable effectiveness between the two treatments. It should be mentioned that intranasal steroids are considered the most effective pharmacological treatment for AR [2]. Furthermore, the pollen intensity was marginally weaker and decreased somewhat faster in the BNS group than in the ATANS group. In contrast to previous findings, LT was the most effective therapy regarding all tested variables in our study. This might be attributable to the fact that the pollen intensity was marginally weaker in the LT group than in the ATANS and BNS groups on day 7.
After 7‑day treatment, the rhinitis symptom score and the endoscopic score decreased significantly from V1 to V2 in the ATANS group. The number of patients who needed no additional medication increased from less than half before treatment to more than half during treatment. Most patients assessed the treatment as good or very good. Taken together, these results indicate that ATANS is an effective non-pharmacological therapy for AR. The consistent pollen intensity in the ATANS group during the 7‑day treatment period provided validity for this conclusion.
Non-pharmacological therapies for AR are of interest to many patients with reservations about antihistamines and corticosteroids. In this study, more patients chose ATANS than BNS and LT (61 vs. 32 and 21 patients, respectively). This suggests that patients with AR prefer non-pharmacological to pharmacological and invasive treatment for AR.
Several studies in the past have sought alternatives to guideline-concordant therapies for AR. The active substances are often naturally-occurring and have physical instead of pharmacological effects. Among such products, newly available on the market are cellulose nasal spray, liposomal nasal spray and ectoine nasal spray combined with eye drops. In double-blind, placebo-controlled studies, nasally applied cellulose powder was shown to be an effective adjunctive treatment to antihistamines for birch pollen allergic children [22] and a comprehensive treatment for grass pollen–sensitive adults [23]. No severe or serious AEs occurred in these trials. The clinical effects of microcrystalline cellulose topical nasal spray on the acute response to allergen challenge were not demonstrated in a randomized, double-blind, placebo-controlled, two-way cross-over clinical trial [24]. However, another NIS indicated that liposomal-based treatment may be a safe and comparable alternative to the guideline-concordant therapy of AR with cromoglycate [25]. Liposomal-based nasal spray as well as the combination of nasal and eye spray improved nasal symptoms by 32 and 21%, respectively, within the 7‑day observation period. In this NIS, we observed a statistically significant improvement in nasal symptoms of 27% in the ATANS group, demonstrating a similar effectiveness of ATANS to the aforementioned non-pharmacological treatment options. In another NIS, ectoine-containing nasal spray and eye drops seemed to be a comparable alternative to traditional anti-inflammatory agents such as azelastine, cromoglycin and levocabastine for the treatment of AR [26, 27]. Patients using ectoine nasal spray showed an improvement of about 32% in the symptoms of rhinorrhoea and 30% in nasal obstruction [26], as well as a reduction of 23% in the total nasal symptom score [27]. These results are in line with those demonstrated in this NIS. Ectoine-containing nasal spray, however, was shown to be inferior to BNS [28].
Taken together, the effectiveness and the safety profiles of ATANS are comparable to those of the aforementioned non-pharmacological treatment options.
Conclusions
This study shows that ATANS is safe and effective in patients with pollen-induced AR. Overall, ATANS can be considered an alternative treatment option for AR patients who wish to avoid side effects of antihistamines or corticosteroids.
Abbreviations
- AE:
-
Adverse event
- AR:
-
Allergic rhinitis
- ARIA:
-
Allergic Rhinitis and its Impact on Asthma
- ATANS:
-
Alpha-tocopherol acetate nasal spray
- BNS:
-
Beclomethasone nasal spray
- EMEA:
-
European Agency for the Evaluation of Medicinal Products
- FDA:
-
Food and Drug Administration
- IQR:
-
Interquartile range
- LT:
-
Loratadine tablets
- N:
-
Number
- NIS:
-
Non-interventional study
- SD:
-
Standard deviation
- V:
-
Visit
References
Pawankar R, Canonica GW, Holgate ST, Lockey RF, Blaiss MS. WAO white book on allergy: update 2013 executive summary. Milwaukee: World Allergy Organization; 2013.
Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63(Suppl 86):8–160. https://doi.org/10.1111/j.1398-9995.2007.01620.x.
Sastre J, Mösges R. Local and systemic safety of intranasal corticosteroids. J Investig Allergol Clin Immunol. 2012;22:1–12.
Church MK, Church DS. Pharmacology of antihistamines. Indian J Dermatol. 2013;58(3):219–24. https://doi.org/10.4103/0019-5154.110832.
Wolthers OD, Stone S, Bareille P, Tomkins S, Khindri S. Knemometry assessment of short-term growth in children with asthma receiving fluticasone furoate for 2 weeks: a randomized, placebo-controlled, crossover trial. Clin Ther. 2017;39(6):1191–9. https://doi.org/10.1016/j.clinthera.2017.04.011.
Skoner DP, Berger WE, Gawchik SM, Akbary A, Qiu C. Intranasal triamcinolone and growth velocity. Pediatrics. 2015;135(2):e348–e56. https://doi.org/10.1542/peds.2014-1641.
Mener DJ, Shargorodsky J, Varadhan R, Lin SY. Topical intranasal corticosteroids and growth velocity in children: a meta-analysis. Int Forum Allergy Rhinol. 2015;5(2):95–103. https://doi.org/10.1002/alr.21430.
Lee LA, Sterling R, Maspero J, Clements D, Ellsworth A, Pedersen S. Growth velocity reduced with once-daily fluticasone furoate nasal spray in prepubescent children with perennial allergic rhinitis. J Allergy Clin Immunol Pract. 2014;2(4):421–7. https://doi.org/10.1016/j.jaip.2014.04.008.
Jauregui I, Mullol J, Davila I, Ferrer M, Bartra J, del Cuvillo A, et al. Allergic rhinitis and school performance. J Investig Allergol Clin Immunol. 2009;19(Suppl 1):32–9.
Bensch GW. Safety of intranasal corticosteroids. Ann Allergy Asthma Immunol. 2016;117(6):601–5. https://doi.org/10.1016/j.anai.2016.06.009.
Naclerio RM. Allergic rhinitis. N Engl J Med. 1991;325(12):860–9. https://doi.org/10.1056/nejm199109193251206.
Zheng K, Adjei AA, Shinjo M, Shinjo S, Todoriki H, Ariizumi M. Effect of dietary vitamin E supplementation on murine nasal allergy. Am J Med Sci. 1999;318(1):49–54.
Fogarty A, Lewis S, Weiss S, Britton J. Dietary vitamin E, IgE concentrations, and atopy. Lancet. 2000;356(9241):1573–4. https://doi.org/10.1016/s0140-6736(00)03132-9.
Shahar E, Hassoun G, Pollack S. Effect of vitamin E supplementation on the regular treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2004;92(6):654–8. https://doi.org/10.1016/s1081-1206(10)61432-9.
Lamprecht J. Alpha-Tocopherolacetat als Nasenspray (Filme nasale®) wirkt bei der allergischen Rhinitis. 86th Annual Meeting of the German Society of Oto-Rhino-Laryngology, Head and Neck Surgery 2015. 2015. https://doi.org/10.3205/15hnod079.
Testa D, Marcuccio G, Panin G, Bianco A, Tafuri D, Thyrion FZ, et al. Nasal mucosa healing after endoscopic sinus surgery in chronic rhinosinusitis of elderly patients: role of topic alpha-tocopherol acetate. Aging Clin Exp Res. 2017;29(Suppl 1):191–5. https://doi.org/10.1007/s40520-016-0647-x.
Sieber J, Shah-Hosseini K, Mosges R. Specific immunotherapy for allergic rhinitis to grass and tree pollens in daily medical practice-symptom load with sublingual immunotherapy compared to subcutaneous immunotherapy. Ann Med. 2011;43(6):418–24. https://doi.org/10.3109/07853890.2011.595426.
Mosges R, Pasch N, Sayar A, Schmalz P, Vent J. Survey of sensory perception and patients’ subjective assessment of the application of nasal sprays—the nasal-spray-sensoric-scale. Laryngorhinootologie. 2009;88(9):587–91. https://doi.org/10.1055/s-0029-1202369.
Akobeng AK. Assessing the Validity of Clinical Trials. J Pediatr Gastroenterol Nutr. 2008;47(3):277–82. https://doi.org/10.1097/MPG.0b013e31816c749.
Krouse JH, Roland PS, Marple BF, Wall GM, Hannley M, Golla S, et al. Optimal duration of allergic rhinitis clinical trials. Otolaryngol Head Neck Surg. 2005;133(4):467–87. https://doi.org/10.1016/j.otohns.2005.07.024. discussion 88.
Bachert C, Borchard U, Wedi B, Klimek L, Rasp G, Riechelmann H et al. [Allergic rhinoconjunctivitis. Guidelines of the DGAI in association with the DDG]. J Dtsch Dermatol Ges. 2006;4(3):264-75. https://doi.org/10.1111/j.1610-0387.2006.04349.x.
Aberg N, Dahl A, Benson M. A nasally applied cellulose powder in seasonal allergic rhinitis (SAR) in children and adolescents; reduction of symptoms and relation to pollen load. Pediatr Allergy Immunol. 2011;22(6):594–9. https://doi.org/10.1111/j.1399-3038.2011.01182.x.
Aberg N, Ospanova ST, Nikitin NP, Emberlin J, Dahl A. A nasally applied cellulose powder in seasonal allergic rhinitis in adults with grass pollen allergy: a double-blind, randomized, placebo-controlled, parallel-group study. Int Arch Allergy Immunol. 2014;163(4):313–8. https://doi.org/10.1159/000360734.
Lansberg MP, DeTineo M, Lane J, Pinto JM, Baroody FM, Naclerio RM. A clinical trial of a microcrystalline cellulose topical nasal spray on the acute response to allergen challenge. Am J Rhinol Allergy. 2016;30(4):269–73. https://doi.org/10.2500/ajra.2016.30.4314.
Bohm M, Avgitidou G, El Hassan E, Mosges R. Liposomes: a new non-pharmacological therapy concept for seasonal-allergic-rhinoconjunctivitis. Eur Arch Otorhinolaryngol. 2012;269(2):495–502. https://doi.org/10.1007/s00405-011-1696-6.
Eichel A, Bilstein A, Werkhäuser N, Mosges R. Meta-analysis of the efficacy of ectoine nasal spray in patients with allergic rhinoconjunctivitis. J Allergy (cairo). 2014;2014:292545. https://doi.org/10.1155/2014/292545.
Werkhäuser N, Bilstein A, Sonnemann U. Treatment of allergic rhinitis with ectoine containing nasal spray and eye drops in comparison with azelastine containing nasal spray and eye drops or with cromoglycic Acid containing nasal spray. J Allergy (Cairo). 2014; https://doi.org/10.1155/2014/176597.
Sonnemann U, Moller M, Bilstein A. Noninterventional open-label trial investigating the efficacy and safety of ectoine containing nasal spray in comparison with beclomethasone nasal spray in patients with allergic rhinitis. J Allergy (Cairo). 2014; https://doi.org/10.1155/2014/297203.
Acknowledgements
We kindly thank the patients who participated in this study. We would also like to thank Yann Reydelet for the valuable data management and Gena Kittel for editing and proofreading the manuscript.
Funding
Sponsorship for this study and article processing charges were provided by PANIN S.R.L. (Rovigo, Italy). All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
U. Pieper-Fürst, V.-A. Dao and K. Shah-Hosseini declare that they have no competing interests. G. Panin is the R&D manager of PANIN S.R.L. (Rovigo, Italy). J. Lamprecht reports personal fees from Hulka S.R.L. (Rovigo, Italy). R. Mösges reports personal fees from ALK, grants from ASIT biotech, personal fees from Allergopharma, personal fees from Allergy Therapeutics, grants and personal fees from Bencard, grants from Leti, grants, personal fees and non-financial support from Lofarma, non-financial support from Roxall, grants and personal fees from Stallergenes, grants from Optima, personal fees from Friulchem, personal fees from Hexal, personal fees from Servier, personal fees from Klosterfrau, non-financial support from Atmos, personal fees from Bayer, non-financial support from Bionorica, personal fees from FAES, personal fees from GSK, personal fees from MSD, personal fees from Johnson & Johnson, personal fees from Meda, personal fees and non-financial support from Novartis, non-financial support from Otonomy, personal fees from Stada, personal fees from UCB, non-financial support from Ferrero, grants from BitopAG, grants from Hulka, personal fees from Nuvo, grants from Ursapharm outside the submitted work.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committees and with the 1964 Declaration of Helsinki, as revised in 2013. Informed consent was obtained from all individual participants included in the study.
Additional information
Authors’ contributions
Ralph Mösges, Giorgio Panin, and Jürgen Lamprecht conceptualized and designed the study. Ursula Pieper-Fürst conducted the trial. Kija Shah-Hosseini performed the statistical analyses. Ursula Pieper-Fürst and Van-Anh Dao wrote the manuscript. All authors approved the final manuscript for submission.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Caption Electronic Supplementary Material
Online Resource 1: Pollen data.
(A) Mean pollen intensity in three treatment groups. (B) Mean daily pollen intensity in three treatment groups. Mean daily pollen intensity in the ATANS group (C), BNS group (D) and LT group (E). Pollen intensity definition: 0 = no pollen, 1 = mild pollen intensity, 2 = moderate pollen intensity, 3 = high pollen intensity
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pieper-Fürst, U., Dao, VA., Shah-Hosseini, K. et al. Alpha-tocopherol acetate nasal spray in the treatment of pollen-induced allergic rhinitis. Allergo J Int 28, 152–159 (2019). https://doi.org/10.1007/s40629-018-0086-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40629-018-0086-7