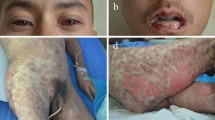
Abstract
Background
In contrast to the classic frequently seen drug eruptions, the rare severe drug reactions such as acute generalized exanthematous pustulosis (AGEP), erythema multiforme (EM), Stevens–Johnson syndrome (SJS), toxic epidermal necrolysis (TEN, Lyell’s syndrome), and drug reaction with eosinophilia and systemic symptoms (DRESS) are frequently associated with increased mortality. Neither their acute management nor their further allergy diagnostic testing to avoid re-exposure and enable restriction of substances prohibited due to the event are standardized.
Materials and methods
The management of severe adverse drug reactions was investigated in a 10-year monocentric retrospective study.
Results
TEN (43.5%) and EM (29.0%) were the two most common subtypes of severe adverse drug reactions, while AGEP (3.2%), SJS (6.5%), and DRESS (17.7%) were less frequent. The acute management of 62 patients with severe adverse drug reactions was generally performed using systemic glucocorticoids (58.1%) or as a combination therapy consisting of glucocorticoids and intravenous immunoglobulins (IVIG, 41.9%), which were usually used in severe clinical courses. The most commonly suspected triggers were beta-lactam antibiotics (28.8%), followed by metamizole (19.4%) and sulfonamide antibiotics (17.7%).
Conclusion
Due to the rarity and heterogeneity of this patient population, there is scant reliable data on the systemic treatment of SJS/TEN. Therefore, whether it confers an evident benefit remains unclear. Although the allergy diagnostic testing of severe adverse drug reactions is complex, it is often able to yield important insights and should be performed.




Similar content being viewed by others
Abbreviations
- AGEP:
-
Acute generalized exanthematous pustulosis
- CsA:
-
Cyclosporine A
- DRESS:
-
Drug reaction with eosinophilia and systemic symptoms
- EM:
-
Erythema multiforme
- GC:
-
Glucocorticoids
- IgE:
-
Immunoglobulin E
- IVIG:
-
Intravenous immunoglobulins
- LTT:
-
Lymphocyte transformation test
- SJS:
-
Stevens–Johnson syndrome
- TEN:
-
Toxic epidermal necrolysis (Lyell’s syndrome)
- ADR:
-
Adverse drug reactions
References
Bork K. Arzneinebenwirkungen. In: Braun-Falco O, Plewig G, Wolff HH, Burgdorf WHC, Landthaler M, editors. Dermatologie und Venerologie. 5th ed. Berlin-Heidelberg: Springer; 2005. pp. 425–6.
Coombs RR. Immunopathology. Br Med J. 1968;1:597–602.
Sidoroff A, Dunant A, Viboud C, Halevy S, Bavinck JN, Naldi L, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP) – results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989–96.
Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92–6.
Mockenhaupt M. Severe drug-induced skin reactions. Stevens-Johnson syndrome and toxic epidermal necrolysis. Hautarzt. 2014;65:415–23.
Kardaun SH, Sekula P, Valeyrie-Allanore L, Liss Y, Chu CY, Creamer D, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071–80.
Rzany B, Mockenhaupt M, Baur S, Schröder W, Stocker U, Mueller J, et al. Epidemiology of erythema exsudativum multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis in Germany (1990–1992): structure and results of a population-based registry. J Clin Epidemiol. 1996;49:769–73.
Chan HL, Stern RS, Arndt KA, Langlois J, Jick SS, Jick H, et al. The incidence of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. A population-based study with particular reference to reactions caused by drugs among outpatients. Arch Dermatol. 1990;126:43–7.
Roujeau JC, Guillaume JC, Fabre JP, Penso D, Fléchet ML, Girre JP. Toxic epidermal necrolysis (Lyell syndrome). Incidence and drug etiology in France, 1981–1985. Arch Dermatol. 1990;126:37–42.
Sekula P, Dunant A, Mockenhaupt M, Naldi L, Bouwes Bavinck JN, Halevy S, et al. Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Invest Dermatol. 2013;133:1197–204.
Roujeau JC, Bastuji-Garin S. Systematic review of treatments for Stevens-Johnson syndrome and toxic epidermal necrolysis using the SCORTEN score as a tool for evaluating mortality. Ther Adv Drug Saf. 2011;2:87–94.
Zimmermann S, Sekula P, Venhoff M, Motschall E, Knaus J, Schumacher M, et al. Systemic Immunomodulating therapies for Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. Jama Dermatol. 2017;153:514–22.
Enk AH, Hadaschik EN, Eming R, Fierlbeck G, French LE, Girolomoni G, et al. European Guidelines (S1) on the use of high-dose intravenous immunoglobulin in dermatology. J Eur Acad Dermatol Venereol. 2016;30:1657–69.
Mahar PD, Wasiak J, Hii B, Cleland H, Watters DA, Gin D, et al. A systematic review of the management and outcome of toxic epidermal necrolysis treated in burns centres. Burns. 2014;40:1245–54.
Ye LP, Zhang C, Zhu QX. The effect of intravenous immunoglobulin combined with corticosteroid on the progression of Stevens-Johnson syndrome and toxic epidermal necrolysis: a meta-analysis. PLoS ONE. 2016;11:e167120.
Valeyrie-Allanore L, Wolkenstein P, Brochard L, Ortonne N, Maitre B, Revuz J, et al. Open trial of ciclosporin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2010;163:847–53.
González-Herrada C, Rodríguez-Martín S, Cachafeiro L, Lerma V, González O, Lorente JA, et al. Cyclosporine use in epidermal necrolysis is associated with an important mortality reduction: evidence from three different approaches. J Invest Dermatol. 2017;137:2092–100.
Finkelstein Y, Macdonald EM, Li P, Hutson JR, Juurlink DN. Recurrence and mortality following severe cutaneous adverse reactions. JAMA. 2014;311:2231–2.
Alniemi DT, Wetter DA, Bridges AG, El-Azhary RA, Davis MD, Camilleri MJ, et al. Acute generalized exanthematous pustulosis: clinical characteristics, etiologic associations, treatments, and outcomes in a series of 28 patients at Mayo Clinic, 1996–2013. Int J Dermatol. 2017;56:405–14.
Möbs C, Pfützner W. Diagnostics of drug hypersensitivity reactions. Hautarzt. 2017;68:19–28.
Paulmann M, Mockenhaupt M. Severe drug-induced skin reactions: clinical features, diagnosis, etiology, and therapy. J Dtsch Dermatol Ges. 2015;13:625–45.
Mashiah J, Brenner S. A systemic reaction to patch testing for the evaluation of acute generalized exanthematous pustulosis. Arch Dermatol. 2003;139:1181–3.
Vaillant L, Camenen I, Lorette G. Patch testing with carbamazepine: reinduction of an exfoliative dermatitis. Arch Dermatol. 1989;125:299.
Barbaud A, Collet E, Milpied B, Assier H, Staumont D, Avenel-Audran M, et al. A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. Br J Dermatol. 2013;168:555–62.
Santiago F, Gonçalo M, Vieira R, Coelho S, Figueiredo A. Epicutaneous patch testing in drug hypersensitivity syndrome (DRESS). Contact Derm. 2010;62:47–53.
Yaylacı S, Demir MV, Temiz T, Tamer A, Uslan MI. Allopurinol-induced DRESS syndrome. Indian J Pharmacol. 2012;44:412–4.
Brockow K, Przybilla B, Aberer W, Bircher AJ, Brehler R, Dickel H, et al. Guideline for the diagnosis of drug hypersensitivity reactions: S2K-guideline of the German Society for Allergology and Clinical Immunology (DGAKI) and the German Dermatological Society (DDG) in collaboration with the Association of German Allergologists (AeDA), the German Society for Pediatric Allergology and Environmental Medicine (GPA), the German Contact Dermatitis Research Group (DKG), the Swiss Society for Allergy and Immunology (SGAI), the Austrian Society for Allergology and Immunology (ÖGAI), the German Academy of Allergology and Environmental Medicine (DAAU), the German Center for Documentation of Severe Skin Reactions and the German Federal Institute for Drugs and Medical Products (BfArM). Allergo J Int. 2015;24:94–105.
Brockow K, Ring J. Anaphylaxis to radiographic contrast media. Curr Opin Allergy Clin Immunol. 2011;11:326–31.
Fernández TD, Torres MJ, Blanca-López N, Rodríguez-Bada JL, Gomez E, Canto G, et al. Negativization rates of IgE radioimmunoassay and basophil activation test in immediate reactions to penicillins. Allergy. 2009;64:242–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J. Ali, B. Schoch, G. Glatthaar, J. Fischer, and A.S. Yazdi declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Ali, J., Schoch, B., Glatthaar, G. et al. Management of severe drug reactions: a retrospective monocentric analysis. Allergo J Int 27, 49–55 (2018). https://doi.org/10.1007/s40629-017-0047-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40629-017-0047-6