Abstract
Objective
Insulin resistance develops due to skeletal muscle inflammation and endoplasmic reticulum (ER) stress. Stachydrine (STA), extracted from Leonurus heterophyllus, has been shown to suppress proliferation and induce apoptosis in breast cancer cells and exert anti-inflammatory properties in the brain, heart, and liver. However, the roles of STA in insulin signaling in skeletal muscle remain unclear. Herein, we examined the impacts of STA on insulin signaling in skeletal muscle under hyperlipidemic conditions and its related molecular mechanisms.
Methods
Various protein expression levels were determined by Western blotting. Levels of mouse serum cytokines were measured by ELISA.
Results
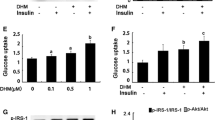
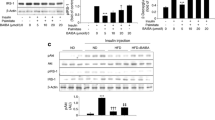
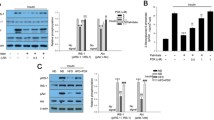
We found that STA-ameliorated inflammation and ER stress, leading to attenuation of insulin resistance in palmitate-treated C2C12 myocytes. STA dose-dependently enhanced AMPK phosphorylation and HO-1 expression. Administration of STA attenuated not only insulin resistance but also inflammation and ER stress in the skeletal muscle of high-fat diet (HFD)-fed mice. Additionally, STA-ameliorated glucose tolerance and insulin sensitivity, as well as serum TNFα and MCP-1, in mice fed a HFD. Small interfering (si) RNA-associated suppression of AMPK or HO-1 expression abolished the effects of STA in C2C12 myocytes.
Conclusions
These results suggest that STA activates AMPK/HO-1 signaling, resulting in reduced inflammation and ER stress, thereby improving skeletal muscle insulin resistance. Using STA as a natural ingredient, this research successfully treated insulin resistance and type 2 diabetes.
Graphical Abstract








Similar content being viewed by others
Abbreviations
- AMPK:
-
AMP-activated protein kinase
- HO-1:
-
Heme oxygenase-1
- ER stress:
-
Endoplasmic reticulum stress
References
Saklayen MG (2018) The global epidemic of the metabolic syndrome. Curr Hypertens Rep 20(2):12
Zheng Y, Ley SH, Hu FB (2018) Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 14(2):88–98
Williams MD, Mitchell GM (2012) MicroRNAs in insulin resistance and obesity. Exp Diabetes Res 2012:484696
Warram JH, Martin BC, Krolewski AS et al (1990) Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med 113(12):909–915
Jove M, Planavila A, Laguna JC et al (2005) Palmitate-induced interleukin 6 production is mediated by protein kinase C and nuclear-factor kappaB activation and leads to glucose transporter 4 down-regulation in skeletal muscle cells. Endocrinology 146(7):3087–3095
Jornayvaz FR, Samuel VT, Shulman GI (2010) The role of muscle insulin resistance in the pathogenesis of atherogenic dyslipidemia and nonalcoholic fatty liver disease associated with the metabolic syndrome. Annu Rev Nutr 30:273–290
Petersen KF, Dufour S, Savage DB et al (2007) The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci U S A 104(31):12587–12594
Zhang C, Shan XL, Liao YL et al (2014) Effects of stachydrine on norepinephrine-induced neonatal rat cardiac myocytes hypertrophy and intracellular calcium transients. BMC Complement Altern Med 14:474
Zhang J, Yang A, Wu Y et al (2018) Stachydrine ameliorates carbon tetrachloride-induced hepatic fibrosis by inhibiting inflammation, oxidative stress and regulating MMPs/TIMPs system in rats. Biomed Pharmacother 97:1586–1594
Zhao L, Wu D, Sang M et al (2017) Stachydrine ameliorates isoproterenol-induced cardiac hypertrophy and fibrosis by suppressing inflammation and oxidative stress through inhibiting NF-kappaB and JAK/STAT signaling pathways in rats. Int Immunopharmacol 48:102–109
Yu N, Hu S, Hao Z (2018) Benificial effect of stachydrine on the traumatic brain injury induced neurodegeneration by attenuating the expressions of Akt/mTOR/PI3K and TLR4/NFkappa-B pathway. Transl Neurosci 9:175–182
Kim TJ, Lee HJ, Pyun DH et al (2021) Valdecoxib improves lipid-induced skeletal muscle insulin resistance via simultaneous suppression of inflammation and endoplasmic reticulum stress. Biochem Pharmacol 188:114557
Liu X, Shan X, Chen H et al (2019) Stachydrine ameliorates cardiac fibrosis through inhibition of angiotensin II/transformation growth factor beta1 fibrogenic axis. Front Pharmacol 10:538
Olivares-Reyes JA, Arellano-Plancarte A, Castillo-Hernandez JR (2009) Angiotensin II and the development of insulin resistance: implications for diabetes. Mol Cell Endocrinol 302(2):128–139
Jung TW, Kim HC, Kim HU et al (2019) Asprosin attenuates insulin signaling pathway through PKCdelta-activated ER stress and inflammation in skeletal muscle. J Cell Physiol 234(11):20888–20899
Jung TW, Lee SH, Kim HC et al (2018) METRNL attenuates lipid-induced inflammation and insulin resistance via AMPK or PPARdelta-dependent pathways in skeletal muscle of mice. Exp Mol Med 50(9):1–11
Lee W, Yun S, Choi GH et al (2018) BAIBA attenuates the expression of inflammatory cytokines and attachment molecules and ER stress in HUVECs and THP-1 cells. Pathobiology 85(5–6):280–288
Lee W, Yun S, Choi GH et al (2018) Fibronectin Type III Domain Containing 4 attenuates hyperlipidemia-induced insulin resistance via suppression of inflammation and ER stress through HO-1 expression in adipocytes. Biochem Biophys Res Commun 502(1):129–136
Kwon CH, Sun JL, Kim MJ et al (2020) Clinically confirmed DEL-1 as a myokine attenuates lipid-induced inflammation and insulin resistance in 3T3-L1 adipocytes via AMPK/HO-1- pathway. Adipocyte 9(1):576–586
Sun JL, Abd El-Aty AM, Jeong JH et al (2020) Ginsenoside Rb2 ameliorates LPS-induced inflammation and ER stress in HUVECs and THP-1 cells via the AMPK-mediated pathway. Am J Chin Med 48(4):967–985
Koves TR, Ussher JR, Noland RC et al (2008) Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7(1):45–56
Foretz M, Even PC, Viollet B (2018) AMPK activation reduces hepatic lipid content by increasing fat oxidation in vivo. Int J Mol Sci. https://doi.org/10.3390/ijms19092826
Thomson DM, Winder WW (2009) AMP-activated protein kinase control of fat metabolism in skeletal muscle. Acta Physiol (Oxf) 196(1):147–154
Daval M, Foufelle F, Ferre P (2006) Functions of AMP-activated protein kinase in adipose tissue. J Physiol 574(Pt 1):55–62
Kim B, Kim MS, Hyun CK (2017) Syringin attenuates insulin resistance via adiponectin-mediated suppression of low-grade chronic inflammation and ER stress in high-fat diet-fed mice. Biochem Biophys Res Commun 488(1):40–45
Boden G (1997) Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 46(1):3–10
Coll T, Eyre E, Rodriguez-Calvo R et al (2008) Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J Biol Chem 283(17):11107–11116
Senn JJ (2006) Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J Biol Chem 281(37):26865–26875
Shi H, Kokoeva MV, Inouye K et al (2006) TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116(11):3015–3025
Kim JK, Kim YJ, Fillmore JJ et al (2001) Prevention of fat-induced insulin resistance by salicylate. J Clin Invest 108(3):437–446
Hu Y, Mao A, Yu Z et al (2016) Anti-endotoxin and anti-inflammatory effects of Chinese herbal medicinal alkaloid ingredients in vivo. Microb Pathog 99:51–55
Yilmaz E (2017) Endoplasmic reticulum stress and obesity. Adv Exp Med Biol 960:261–276
Li M, Zhang Y, Cao Y et al (2018) Icariin ameliorates palmitate-induced insulin resistance through reducing thioredoxin-interacting protein (TXNIP) and suppressing ER stress in C2C12 myotubes. Front Pharmacol 9:1180
Hardie DG, Schaffer BE, Brunet A (2016) AMPK: an energy-sensing pathway with multiple inputs and outputs. Trends Cell Biol 26(3):190–201
Woods A, Williams JR, Muckett PJ et al (2017) Liver-specific activation of AMPK prevents steatosis on a high-fructose diet. Cell Rep 18(13):3043–3051
Musi N, Goodyear LJ (2003) AMP-activated protein kinase and muscle glucose uptake. Acta Physiol Scand 178(4):337–345
Maines MD (1997) The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol 37:517–554
Tenhunen R, Marver HS, Schmid R (1968) The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A 61(2):748–755
Trakshel GM, Kutty RK, Maines MD (1986) Purification and characterization of the major constitutive form of testicular heme oxygenase. The noninducible isoform. J Biol Chem 261(24):11131–11137
Xin G, Du J, Wang YT et al (2014) Effect of oxidative stress on heme oxygenase-1 expression in patients with gestational diabetes mellitus. Exp Ther Med 7(2):478–482
Takahashi T, Shimizu H, Morimatsu H et al (2007) Heme oxygenase-1: a fundamental guardian against oxidative tissue injuries in acute inflammation. Mini Rev Med Chem 7(7):745–753
Liu XM, Peyton KJ, Ensenat D et al (2005) Endoplasmic reticulum stress stimulates heme oxygenase-1 gene expression in vascular smooth muscle. Role in cell survival. J Biol Chem 280(2):872–877
Le WD, Xie WJ, Appel SH (1999) Protective role of heme oxygenase-1 in oxidative stress-induced neuronal injury. J Neurosci Res 56(6):652–658
Jung TW, Kim HC, Abd El-Aty AM et al (2017) Protectin DX suppresses hepatic gluconeogenesis through AMPK-HO-1-mediated inhibition of ER stress. Cell Signal 34:133–140
Minnich A, Tian N, Byan L et al (2001) A potent PPARalpha agonist stimulates mitochondrial fatty acid beta-oxidation in liver and skeletal muscle. Am J Physiol Endocrinol Metab 280(2):E270-279
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. NRF-2021R1F1A1050004 and No. 2022R1A2B5B01001453).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We confirm that there are no known conflicts of interest associated with this publication.
Ethical approval
Animal experiments were approved by the Institutional Animal Care and Use Committee of Chung-Ang University, Seoul, Republic of Korea (Approval No.: 2020-00048) and carried out according to the Guide for the Care and Use of Laboratory Animals (NIH publication, 8th edition, 2011).
Informed consent
For this type of study formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
40618_2022_1866_MOESM1_ESM.tif
Supplementary file1. Figure S1. STA does not affect palmitate-induced TG accumulation and does not modulate insulin-stimulated ERK1/2 signaling. A Western blotting of phosphorylated ERK1/2 expression in C2C12 myocytes treated with palmitate (200 μM) and/or STA (20 μM) for 24 h. Human insulin (5 nM) was used to upregulate insulin signaling for 3 min. B Oil red O-based TG measurement in C2C12 myocytes in the presence of palmitate (200 μM) and/or STA (0-20 μM) for 24 h. Means ± SEM were calculated from three independent experiments. Significance (P < 0.05) *: vs insulin-treated control or control. (TIF 117 KB)
Rights and permissions
About this article
Cite this article
Jung, T.W., Kim, H., Park, S.Y. et al. Stachydrine alleviates lipid-induced skeletal muscle insulin resistance via AMPK/HO-1-mediated suppression of inflammation and endoplasmic reticulum stress. J Endocrinol Invest 45, 2181–2191 (2022). https://doi.org/10.1007/s40618-022-01866-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-022-01866-8