Abstract
Purpose
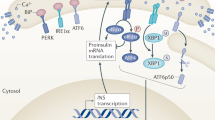
Endoplasmic reticulum (ER) stress is implicated in the development of type 2 diabetes mellitus (T2DM) and insulin resistance. Tribbles homolog 3 (TRB3) is a pseudokinase upregulated by ER stress and hyperglycemia. Glucose-regulated protein 78 (GRP78) is an ER stress protein that is overexpressed under ER stress conditions. The current study aimed to investigate serum levels of TRB3 and GRP78, as an ER stress marker, in T2DM patients and their correlations with the metabolic profile.
Methods
Fifty-seven patients with type 2 diabetes and 23 healthy control subjects were evaluated for serum concentrations of TRB3, GRP78, and AGEs by enzyme-linked immunosorbent assay (ELISA). Fasting plasma glucose (FPG), HbA1c, lipid profile, TNF-α and insulin were also measured, and insulin resistance was calculated using a homeostasis model assessment of insulin resistance (HOMA-IR).
Results
Serum concentrations of TRB3, GRP78, AGEs, and TNF-α were significantly higher in T2DM patients compared to the healthy controls. Moreover, a statistically significant positive correlation was observed between plasma concentrations of TRB3 and FPG, HbA1c, HOMA-IR, and AGE. GRP78 levels were positively correlated with HbA1c and AGEs. There was also a positive correlation between GRP78 and TRB3. AGEs levels were positively correlated with the levels of FPG, HbA1c, HOMA-IR, and TNF-α.
Conclusion
The current findings suggest that TRB3 and GRP78 may contribute to the pathogenesis of T2DM and might be considered as a therapeutic targets for the treatment of this disease.

Similar content being viewed by others
References
Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444(7121):840–846
Nakatani Y et al (2005) Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem 280(1):847–851
Xu J et al (2012) Endoplasmic reticulum stress and diabetic cardiomyopathy. Exp Diabetes Res 2012:5
Ohoka N et al (2005) TRB3, a novel ER stress-inducible gene, is induced via ATF4–CHOP pathway and is involved in cell death. EMBO J 24(6):1243–1255
Tang M et al (2008) Differential regulation of collagen types I and III expression in cardiac fibroblasts by AGEs through TRB3/MAPK signaling pathway. Cell Mol Life Sci 65(18):2924–2932
Wang M et al (2017) TRB3 mediates advanced glycation end product-induced apoptosis of pancreatic β-cells through the protein kinase C β pathway. Int J Mol Med 40(1):130–136
Du K et al (2003) TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 300(5625):1574–1577
Koo S-H et al (2004) PGC-1 promotes insulin resistance in liver through PPAR-α-dependent induction of TRB-3. Nat Med 10(5):530–534
Nourbakhsh M et al (2017) Evaluation of plasma TRB3 and sestrin 2 levels in obese and normal-weight children. Child Obes 13(5):409–414
Lee AS (2001) The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci 26(8):504–510
Khadir A et al (2016) Physical exercise alleviates ER stress in obese humans through reduction in the expression and release of GRP78 chaperone. Metabolism 65(9):1409–1420
Lee ASJCR (2007) GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res 67(8):3496–3499
Wang M et al (2009) Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal 11(9):2307–2316
Kim JH et al (2018) Endoplasmic reticulum chaperone GRP78 regulates macrophage function and insulin resistance in diet-induced obesity. FASEB J 32(4):2292–2304
Liñares-Pose L et al (2018) Genetic targeting of GRP78 in the VMH improves obesity independently of food intake. Genes 9(7):357
Gonzalez-Gronow M et al (2009) GRP78: a multifunctional receptor on the cell surface. Antioxid Redox Signal 11(9):2299–2306
Rossi G, Association AD (2018) Diagnosis and classification of diabetes mellitus. Diabetes Care 33(Suppl 1):S62–S69
Bergman RN, Finegood DT, Ader M (1985) Assessment of insulin sensitivity in vivo. Endocr Rev 6(1):45–86
Keskin M et al (2005) Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics 115(4):e500–e503
Boden G et al (2008) Increase in endoplasmic reticulum stress–related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes 57(9):2438–2444
Özcan U et al (2004) Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306(5695):457–461
Flamment M et al (2012) New insights into ER stress-induced insulin resistance. Trends Endocrinol Metab 23(8):381–390
Wang D et al (2006) Endoplasmic reticulum stress increases glucose-6-phosphatase and glucose cycling in liver cells. Endocrinology 147(1):350–358
Xu L, Spinas GA, Niessen M (2010) ER stress in adipocytes inhibits insulin signaling, represses lipolysis, and alters the secretion of adipokines without inhibiting glucose transport. Hormone Metabolic Res 42(9):643–651
Corcoran CA et al (2005) Genotoxic and endoplasmic reticulum stresses differentially regulate TRB3 expression. Cancer Biol Therapy 4(10):1063–1067
Sowers JRJD (2012) Role of TRIB3 in diabetic and overnutrition-induced atherosclerosis. Diabetes 61(2):265–266
Ti Y et al (2011) TRB3 gene silencing alleviates diabetic cardiomyopathy in a type 2 diabetic rat model. Diabetes 60(11):2963–2974
Liu J et al (2010) Mammalian Tribbles homolog 3 impairs insulin action in skeletal muscle: role in glucose-induced insulin resistance. Am J Physiol Endocrinol Metabol 298(3):E565–E576
Oberkofler H et al (2010) Aberrant hepatic TRIB3 gene expression in insulin-resistant obese humans. Diabetologia 53(9):1971–1975
Gong H-P et al (2009) TRIB3 functional Q84R polymorphism is a risk factor for metabolic syndrome and carotid atherosclerosis. Diabetes Care 32(7):1311–1313
Prudente S et al (2005) The functional Q84R polymorphism of mammalian Tribbles homolog TRB3 is associated with insulin resistance and related cardiovascular risk in Caucasians from Italy. Diabetes 54(9):2807–2811
Zhang W et al (2016) Skeletal muscle TRIB3 mediates glucose toxicity in diabetes and high-fat diet-induced insulin resistance. Diabetes 65(8):2380–2391
Qian B et al (2008) TRIB3 is implicated in glucotoxicity-and oestrogen receptor-stress-induced b-cell apoptosis. J Endocrinol 199(3):407
Rutkowski DT, Kaufman RJ (2004) A trip to the ER: coping with stress. Trends Cell Biol 14(1):20–28
Zhang K, Kaufman RJ (2004) Signaling the unfolded protein response from the endoplasmic reticulum. J Biol Chem 279(25):25935–25938
Sharma NK et al (2008) Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. J Clin Endocrinol Metab 93(11):4532–4541
Kammoun HL et al (2009) GRP78 expression inhibits insulin and ER stress–induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Investig 119(5):1201–1215
Zhao Y et al (2015) The dynamic changes of endoplasmic reticulum stress pathway markers GRP78 and CHOP in the hippocampus of diabetic mice. Brain Res Bull 111:27–35
Laybutt D et al (2007) Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 50(4):752–763
Zhang L et al (2009) GRP78, but not protein-disulfide isomerase, partially reverses hyperglycemia-induced inhibition of insulin synthesis and secretion in pancreatic β-cells. J Biol Chem 284(8):5289–5298
Lindenmeyer MT et al (2008) Proteinuria and hyperglycemia induce endoplasmic reticulum stress. J Am Soc Nephrol 19(11):2225–2236
Van Krieken R et al (2019) Cell surface expression of 78-kDa glucose-regulated protein (GRP78) mediates diabetic nephropathy. J Biol Chem 294(19):7755–7768
Girona J et al (2019) The circulating GRP78/BiP is a marker of metabolic diseases and atherosclerosis: bringing endoplasmic reticulum stress into the clinical scenario. J Clin Med 8(11):1793
Ma N et al (2020) Expression of serum GRP78 and CHOP in endoplasmic reticulum stress pathways of chinese type 2 diabetic kidney disease patients (preprint)
Diaz-Morales N et al (2018) Does metformin modulate endoplasmic reticulum stress and autophagy in type 2 diabetic peripheral blood mononuclear cells? Antioxid Redox Signal 28(17):1562–1569
Teodoro-Morrison T et al (2013) GRP78 overproduction in pancreatic beta cells protects against high-fat-diet-induced diabetes in mice. Diabetologia 56(5):1057–1067
Adamopoulos C et al (2014) Advanced glycation end-products induce endoplasmic reticulum stress in human aortic endothelial cells. Clin Chem Lab Med 52(1):151–160
Özcan U et al (2006) Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313(5790):1137–1140
Bañuls C et al (2017) Oxidative and endoplasmic reticulum stress is impaired in leukocytes from metabolically unhealthy vs healthy obese individuals. Int J Obesity 41(10):1556–1563
Jousse C et al (2007) TRB3 inhibits the transcriptional activation of stress-regulated genes by a negative feedback on the ATF4 pathway. J Biol Chem 282(21):15851–15861
Gaspar RC et al (2020) Aging is associated with increased TRB3, ER stress, and hepatic glucose production in the liver of rats. Exp Gerontol 139:111021
Su X-D et al (2011) Elevated serum levels of advanced glycation end products and their monocyte receptors in patients with type 2 diabetes. Arch Med Res 42(7):596–601
Uribarri J et al (2015) Elevated serum advanced glycation endproducts in obese indicate risk for the metabolic syndrome: a link between healthy and unhealthy obesity? J Clin Endocrinol Metab 100(5):1957–1966
Hachiya H et al (2014) Advanced glycation end products impair glucose-induced insulin secretion from rat pancreatic β-cells. J Hepatobiliary Pancreat Sci 21(2):134–141
Shu T et al (2011) AGEs decrease insulin synthesis in pancreatic β-cell by repressing Pdx-1 protein expression at the post-translational level. PLoS ONE 6(4):e18782
Pinto-Junior DC et al (2018) Advanced glycation end products-induced insulin resistance involves repression of skeletal muscle GLUT4 expression. Sci Rep 8(1):1–11
Lily M, Godwin MJCFP (2009) Treating prediabetes with metformin: systematic review and meta-analysis. Can Family Phys 55(4):363–369
Jung TW et al (2012) Metformin prevents endoplasmic reticulum stress-induced apoptosis through AMPK-PI3K-c-Jun NH2 pathway. Biochem biophys Res 417(1):147–152
Acknowledgements
This study was financially supported by a grant from Endocrinology and Metabolism Research Institute, Tehran University of Medical Sciences (grant number 1396-02-97-2170) and a grant from Iran University of Medical Science (grant number 33203).
Author information
Authors and Affiliations
Contributions
RS conducted and supervised the study design and contributed to the writing of the manuscript. M.N. conducted the study and edited the manuscript. NH and MN contributed to sample collection and preparation SEM and HZ performed the immunoassay experimental work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Ethical approval
This study was performed in compliance with the ethical standards of the Declaration of Helsinki and Iran University of Medical Sciences. The study was approved by Ethics Committee of Iran University of Medical Sciences and Endocrinology and Metabolism Research Institute of Tehran University of Medical Sciences.
Informed consent
All subjects gave their written informed consent before participating in the study.
Data availability statement
The data and analysis of the present study are available from the corresponding author on reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nourbakhsh, M., Sharifi, R., Heydari, N. et al. Circulating TRB3 and GRP78 levels in type 2 diabetes patients: crosstalk between glucose homeostasis and endoplasmic reticulum stress. J Endocrinol Invest 45, 649–655 (2022). https://doi.org/10.1007/s40618-021-01683-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-021-01683-5