Abstract
Aging and menopause are associated with morphological and functional changes which may lead to loss of muscle mass and therefore quality of life. Resistance training (RT) is an effective training mode to increase muscle mass. We reviewed the existing literature to identify studies implementing RT protocols and evaluating muscle hypertrophy exclusively in healthy, postmenopausal and elderly women. Participants’ age range was comprised between 50 and 80 years. The primary outcome observed was muscle hypertrophy. Fat mass was also evaluated, if available. PubMed and Web of Science were the screened database, and original articles written in English and published from 2000 up to 2020 were included. 26 articles were considered eligible and included. Quality assessment revealed a “moderate quality” of the included studies, however the majority of studies was able to reach level 4 of evidence and on overall grade of recommendation C. In total, data from 745 female participants subjected to different forms of resistance training were considered. Heterogeneity across studies was present regarding study design, intervention length (mean 16 weeks), training frequency (3 d/w), no. of exercises (n = 7.4) and participants’ age (65.8 ± 4.9 years). Small-to-moderate significant increases (k = 43; SMD = 0.44; 95% CI 0.28; 0.60; p < 0.0001) of lean body mass were observed in post-menopausal and elderly women, regardless of age, intervention period, weekly training frequency and no. of exercises. No effects were noted for fat mass (k = 17; SMD = 0.27; 95% CI − 0.02; 0.55; p = 0.07). Studies need to concentrate on providing information regarding training parameters to more effectively counteract the effects of aging and menopause on skeletal muscle mass.
Similar content being viewed by others
Introduction
Muscle hypertrophy is defined as an increase in the cross-sectional area of a muscle due to the increase in muscle protein synthesis and contractile tissue [1, 2]. Muscle hypertrophy is a multifaceted phenomenon, that is founded on mechanical stimulation, as well as metabolic and endocrine processes that have been shown to impact gene transcription via different signaling pathways [2]. Mechanical loading, in particular, leads to a number of intracellular actions that ultimately regulate gene expression and protein synthesis via mTORC1 pathway activation [3,4,5]. Therefore, an effective strategy to promote muscle hypertrophy is represented by long-term resistance training (RT) in both men and women of different ages [6,7,8]. Variables such as exercise intensity, exercise frequency, rest periods and training volume can be manipulated in order to maximize the magnitude of the effect on muscle hypertrophy and strength [3]. Recent studies have shown that muscle hypertrophy is also associated with strength gains not only in young and middle aged adults, but also in older men and women [9]. This is of particular importance since declines in muscle mass and strength are observed due to aging [10, 11]. The age-related loss of muscle mass and strength can lead to physical disability and frailty [12, 13] and overall is associated with an increased risk of falls [14,15,16]. Multiple studies have found a link between low levels of muscle mass and low functional capacity [17, 18]. In addition, since muscle is a very metabolically active tissue, metabolic disorders associated with aging, such as diabetes, osteoporosis or decrease in testosterone and growth hormone levels may frequently occur [19, 20]. Therefore, muscle mass loss represents a significant problem for older adults.
Muscle mass loss is also associated to menopause, since a physiological hormonal change is present due to menstruation cessation [21]. In particular, a decline in estrogen concentration has detrimental effects on skeletal muscle mass and functionality, leading to reduced bone mass density, redistribution of fat to the visceral area and increased risk of cardiovascular events [21, 22]. Notably, post-menopausal women with reduced skeletal muscle mass have a 2.1 higher risk of falling and a 2.7 times greater risk of sustaining a fracture compared to women with preserved muscle mass [23].
RT can be used as a potential method of offsetting decline in muscle mass and strength, as improvements in muscle mass have been detected in postmenopausal, middle-aged and older women after RT [24,25,26,27,28].
To our knowledge, despite abundant evidence with regards to variance in response to RT in men and women, there are not enough original investigations able to provide specific guidelines for post-menopausal and elderly women in order to optimize maximal muscle gains. Thus, the main objective of this study is to review the existing literature to identify and analyze current evidence with regards to RT protocols aiming to induce muscle hypertrophy in the post-menopausal and elderly population.
Methods
The manuscript followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [29].
Search strategy and study selection
The databases PubMed (NLM), Web of Science (TS) and Scopus were used to perform a comprehensive search for relevant articles published between January 1, 2000 and November 11, 2020. The search strategy included terms in the search field “title” and/or “topic” and “abstract” of each database. The final searches were then executed using the appropriate specifications of each database using the PICOS format (See supplementary file).
Eligibility criteria
To be included studies: (1) had to include healthy women aged between 50 and 80 years of age, (2) with no physical, mental or neurological disorders, (3) interventions based solely on RT programs conducted in postmenopausal and older adult women, (4) pre and post-intervention results and (5) published in English. Publications were excluded if: (1) Reviews, meta-analysis, abstracts, scientific conference works, posters, citations, letters to the editor, books, statements, (2) non-peer reviewed journal articles, (3) commentaries, (4) together with studies reported in languages other than English.
The primary outcome was identified in a change in muscle mass, i.e. hypertrophy measured by dual-energy X-ray absorptiometry (DXA), magnetic resonance imaging (MRI), ultrasound imaging (USI), bioelectric impedance analysis (BIA) or other valid method able to detect changes in lean tissue.
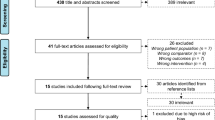
Study record
Search results were uploaded to EndNote X 8.1 (Clarivate Analytics, Jersey, UK) and duplicates were removed. Two independent investigators (ET and AG) screened the titles and abstracts for relevance based on inclusion criteria for this systematic review. Full text of articles were also screened if title and abstract were not sufficient to determine eligibility. Disagreement of article inclusion was resolved by discussion and consensus with a third investigator (AB). The screening process has been summarized in a PRISMA flow diagram (Fig. 1). Three tables were created to extract relevant study data using a Microsoft Excel (Microsoft Corp, Redmond, Washington) spreadsheet. In the first table, information on the first author and year of publication, sample size, mean age and standard deviation, exercise intensity, duration of the intervention, and exercise frequency per week were shown. The second table consisted of test battery used, pre-intervention values, post-intervention values and potential discrepancy (possible incremental change) regarding lean body mass. The third table included data relevant to fat mass, when available. Authors were contacted via email if important data was missing from a particular study. If the contacted author did not respond to the questions asked about the specifics of a study, these were excluded from the review. The WebPlotDigitizer (version 4.2) software, was used to extrapolate information from figures, if relevant information for this review was not included in tables or the main text of the manuscripts.
Risk of bias assessment
For risk of bias assessment, we used The Downs and Black checklist [30] which assesses the quality of original research articles in order to synthesize evidence from quantitative studies for public health purposes. This checklist contains 27 ‘yes’-or- ‘no’ questions across five domains. It provides both an overall score for study quality and a numeric score out of a possible 32 points. The five domains comprise questions concerning study quality, external validity, study bias, confounding and selection bias, and power of the study.
Two independent researchers completed the Downs and Black checklist (ET and AG) for included articles to determine the quality of each study. The maximum score a study can receive is 32, with higher scores denoting greater quality. The studies were then separated into groups and labeled as ‘high quality’ (score 23–32), ‘moderate quality’ (score 19–22), ‘lower quality’ (score 15–18) or ‘poor quality’ (≤ 14). Interclass correlation statistical method was used to determine inter-rater reliability. Quality of evidence was obtained by the study design and by the Downs and Black score (Supplementary File). Levels of evidence and grades of recommendation have been also included for each study. The guidelines from the Centre for Evidence-Based Medicine (CEBM, http://www.cebm.net, Last accessed 12/02/2021) regarding the grading for evidence and the guidelines of the American Society of Plastic Surgeons (https://www.plasticsurgery.org/documents/medical-professionals/health-policy/evidence-practice/ASPS-Scale-for-Grading-Recommendations.pdf, last accessed 12/02/2021) regarding grading of recommendation were adopted. A supplementary table has been provided with the results of the quality assessment (Supplementary File).
Data synthesis
The included studies were first synthesized through a narrative description of the features deriving from each study. Afterwards, the essential characteristics of the studies were represented in tables, where means, standard deviations and the percentage difference between pre- and post- condition were reported. Descriptive statistics of the studies was performed through Jamovi (version 1.6.3.0, The jamovi project, 2020).
Concerning the metanalytic synthesis, the considered outcomes were body lean mass and body fat mass. For each study, means and standard deviations were noted, together with the assessment method used to detect the outcomes (BIA, DXA, MRI, US).
Meta-analysis was performed through the package metafor of the R software (version 3.5.3), using the random effect model on the Standardized Mean Difference (SMD) between pre- and post- measurements. The effects were then represented through a forest plot, and to detect potential influences of publication bias, a funnel plot was performed. The heterogeneity of the studies was estimated through the Cochrane’s Q, and a moderator analysis with age (defined by two categories: below 65 and above 65 years of age), intervention length, number of weekly sessions and number of exercises proposed was planned. Finally, to detect the validity of the results included in the meta-analysis, two sensitivity analyses considering measurement tool and study quality were performed. In the first one, only results derived from DXA were included in the meta-analysis, while in the second one, the effects from poor quality studies were considered.
Results
Search outcomes
The electronic database search yielded 7816 articles (Pubmed = 3880, Scopus = 2159, Web Of Science = 1777). Ten additional articles were identified from other sources as potentially relevant. A total of 7459 irrelevant articles were excluded based on title and abstract and further 269 duplicate records were removed. Preliminary search results and duplicate removal provided a total of 88 articles. First steps in the initial assessment of articles were to screen in detail the titles and the abstracts to identify only relevant articles. Subsequently, these full-text articles were screened for relevance and during this process, 41 articles were removed since these were not eligible according to the inclusion criteria. Twenty one articles were excluded from the study, mainly due to inadequate exposure during the intervention, meaning that methods other than RT were incorporated in the study, or ineligible outcomes were detected which did not fit the inclusion criteria of the review. Finally, 26 articles met the inclusion criteria and were included in the study [9, 31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55]. Amongst included studies, the average number of participants per study was 23 while the mean duration of the studies was 16 weeks. On average, participants underwent 3 RT sessions per week including 7.5 exercises, at an intensity of ⁓60% of their 1RM, performing between 9 and 16 repetitions per set. Articles differed greatly in terms of study design, intervention length, follow-up period, subjects’ age, observed outcomes and measurement of the main outcomes.
Table 1 provides a summary of the studies included in the review. All the included records were original research articles (n = 26). In total, data from 745 participants were pooled for this review. Studies ranged from 8 [31] to 78 participants [54]. The included articles were published over 20 years from 2000 to 2020.
In regards to the measured outcomes, of the 26 articles included in the study, eighteen articles used DXA scanners [33, 34, 36, 38,39,40,41,42,43,44,45,46, 48,49,50, 52, 54] to determine whether muscle hypertrophy was evident or not after the intervention, four articles used a BIA [35, 51, 53, 55], two articles used MRI [9, 31] scans and two articles used USI [32, 37]. It is important to note that these screening tools were used to measure different regions of the body which had been exposed to the RT.
Of the different body regions exposed to RT, 23 studies implemented full-body RT [32,33,34,35,36,37,38,39,40,41, 43,44,45,46,47,48,49,50,51,52,53,54,55], and 3 implemented lower body RT [9, 31, 42]. Thus, only the areas of the body which underwent the RT intervention have been examined by the afore mentioned screening tools.
Table 2 provides a summary of the results of the primary outcomes of interest, while Table 3 provides measures of body fat mass when these were available. Pre and post-intervention values have been identified for each study, and differences between the two have been outlined, when possible. As for the testing methods, there was high heterogeneity in terms of reporting interventions. Some studies reported absolute values, others the percentage of lean bone-free muscle tissue while others reported it as a muscle mass index. Alternatively, some studies reported kilograms of muscle mass prior and after the intervention. Therefore, in order to quantify and normalize the effect of each intervention, percentage differences were determined. A mean increase of 4.8% of lean body mass and a mean decrease of 2.1% of fat body mass have been observed across the retrieved studies.
Risk of bias assessment
Risk of bias assessment was completed for all included articles. The mean Downs and Black checklist score was 19.8 with the range between 14 and 27. Studies were then divided following different quality categories suggested by Tremblay et al. [56]. Six studies were placed into ‘high quality’ category, twelve studies were placed into ‘moderate quality’ category, six studies were placed into ‘lower quality’ category and two studies ware placed into poor quality category. The inter rater reliability coefficient between the assessors was 0.87, which as reported by Dawson and Trapp [57] corresponds to “very good agreement”. For detailed risk of bias reporting of each study, refer to Supplementary File. Level of evidence of the included studies ranged between 1B and 4, with 2 studies reaching level 1B, 6 studies reaching level 2B, 2 study reaching level 3B and 16 studies reaching level 4, suggesting an overall grade C of recommendation of the included manuscripts. A breakdown for each study is provided in Supplementary File.
Meta-analytic synthesis of results
The meta-analysis was performed on lean body mass and fat body mass. First of all, funnel plots did not show any publication bias (Fig. 2 shows lean body mass). Concerning lean body mass, a significant small-to-medium increase in the post-measurement was observed across k = 43 effects (SMD = 0.44; 95% CI 0.28; 0.60; p < 0.0001) (Fig. 3). Heterogeneity of the study resulted significant, with a Q(df = 42) = 109.95, p = 0.0001. Regarding fat body mass, no significant effect was detected across k = 17 effects (SMD = 0.27; 95% CI − 0.02; 0. 55; p = 0.07) (Fig. 4). Since the retrieved effects were homogeneous, we decided to calculate moderator analysis through mixed effects model. A moderator analysis was performed concerning age of the participants (defining two categories: below 65 and above 65 years of age), intervention length, number of weekly sessions and number of exercises proposed, highlighting no significant difference in the retrieved effects, neither for lean nor fat body mass.
Figure shows the forest plot of the meta-analytic results regarding lean body mass. 2 Twice a week, 3 three times a week, BL bilateral, DT-ReT detraining-retraining, DUP ondulating periodized, HS High supervised, HV high volume, LI-BFR low load blood flow restriction exercise, LV low volume, MH moderate to high intensity elastic band resistance exercise, MJ multi joint, MS multiple-set resistance training, MV muscle volume, NP non periodized, PR pyramid, RF rectus femoris, RT resistance training, SJ single joint, SS single-set resistance training, TD traditional, UL unilateral, VL vastus lateralis, VHS very high supervised; < 5% < 5% of truck fat gain
Figure shows the forest plot of the meta-analytic results regarding fat body mass. 2 Twice a week, 3 three times a week, ALT alternating upper and lower body, DUP periodized, MJ-SJ multi to single joint, NP non periodized, RT resistance training, SJ-MJ single to multi joint; < 5% < 5% of truck fat gain
The results of the sensitivity analyses showed that considering only results from DXA returned a SMD = 0.36 (95% CI 0.19–0.53, p < 0.0001), therefore very similar to our main results. The second sensitivity analysis was performed excluding the poor-quality studies, with a SMD = 0.45 (95% CI 0.27–0.63, p < 0.0001), indicating that poor-quality studies did not affect the average effect size.
Discussion
This review article aimed to identify and analyze manuscripts regarding RT and hypertrophic responses in a postmenopausal and elderly adult female population. Our main findings highlight that all the analyzed RT protocols were able to moderately increase muscle mass in the sampled populations, despite differences in intervention length and assessment procedures. These effects however, are small-to-moderate (SMD = 0.44). Interestingly, no difference in lean body mass increase was present regarding age, weekly frequency and intervention length. Therefore, we could not identify a minimum dose–response for lean muscle mass improvement in the retrieved studies regarding RT. A systematic review and meta-analysis by Schoenfeld et al. [58] aimed to identify the main training parameters in order to increase strength and hypertrophic adaptations in a general population. The study has concluded that intensity is determinant in strength increases while volume can be modulated over different spectrums to promote muscle hypertrophy. These findings may provide explanation to our main results, since the included protocols had similar intensities and volume (being that the majority of studies were performing between 8 and 12 repetitions with an intensity of around 60% of 1RM). Another meta-analysis [59] which evaluated the training frequency of RT programs on gains in muscular strength has concluded that increased frequency is linked to increases in strength. However, when age groups were analyzed, only young adults seemed to benefit from increased RT frequency, while older adults did not. However, the results of this latter study only took into account measures of strength and not muscle mass. Nevertheless, evidence of a dose–response relationships in the elderly (taking into account both male and female) exists, suggesting that 2 sessions per week, performing 2 to 3 sets of 8 exercises, is effective in promoting strength and to modify muscle morphology [60]. The suggested protocol almost overlaps the mean reported data present in Table 1, which could explain the homogeneity of the results regarding lean body mass improvements observed across the retrieved studies.
It is important to note that increased muscle mass does not necessarily imply a causal relation with strength improvements [61] since the mechanisms responsible for strength development and muscle hypertrophy are different in nature [62, 63]. For example strength improvements as a result of increased neural drive are observed well before muscle hypertrophy as the result of increased motor unit firing rate or agonist–antagonist co-activation [62], while muscle hypertrophy is mainly stimulated by metabolic stress and mechanical tension which then activate intracellular pathways inducing muscle growth[63]. Although not a primary outcome of this review, as reported in Table 2, increases in strength were also observed for bench press, chest press, leg press and knee extension exercises.
The small effects highlighted by the meta-analytic synthesis, seem to be in line with the most recent scientific evidence, since as a consequence of aging, increased anabolic resistance, diminished muscle regeneration, impaired muscle activation and a reduction of the number of motor units are frequently observed [64]. However, precisely for these reasons it is important to engage postmenopausal and elderly women in RT programs, in order to improve muscle mass and strength, to reduce the risk of injury, and improve quality of life during the aging process [65].
Other analyzed aspect in this review was fat mass, which did not show any difference as a consequence of RT. Despite the general agreement regarding improved muscle mass to RT, there are still controversial reportings regarding fat mass [66], since some authors advocate decreases [67], while others do not [35] in this specific population.
It is important to outline that only the study by Nascimento et al. [34] considered dietary intake along with the RT protocol. Although nutritional aspects of training and recovery go outside the scope of this study, we cannot neglect the importance of proper diet in muscle hypertrophy [68], especially for post-menopausal women in which a correct nutritional regimen is recommended [21, 69], since the frequent dyslipidemic profiles observed [66]. In addition to nutritional status, other important consideration needs to be outlined regarding hormonal replacement therapy that may be prescribed as a form of prevention for estrogen deficiency. None of the included participants were undergoing hormonal therapy. To be noted that six studies [32, 37, 40, 50, 52, 53] did not specify if the participants were using hormonal replacement therapy. Therefore, the results of this study may primarily be attributable to the effects of the RT interventions.
Concerning clinical application, Nelson [70] and colleagues have shown that adults who do not take part in regular RT lose on average 0.46 kg of muscle per year from the age of 50. Additionally, sedentary subjects have reported a 50% reduction in fast-twitch muscle fibers by the age of 80 [27, 71]. These review findings are particularly important for post-menopausal and older women who are more susceptible to sarcopenia than men [72, 73]. Thus, creating exercise prescription that will induce hypertrophy, specifically in postmenopausal and older women could represent an efficient strategy to counteract the effects of sarcopenia and would contribute to overall better quality of life in adult women [74].
The strengths of this review embedded a comprehensive search strategy, stringent predetermined inclusion and exclusion criteria and thorough analyses of each article included. We included articles that examined direct and indirect measures of muscle hypertrophy and focused on incremental changes in the musculature trained in postmenopausal and older adult women. The results of our study provide evidence that RT may possibly counteract the effects of sarcopenia.
Nevertheless, our study is not without limitations, including the various types of outcome measurements sampled with heterogeneous methods. Despite significant efforts to identify appropriate techniques for muscle mass quantification [75, 76], a consensus regarding a gold standard procedure still needs to be defined. A recent review article has proposed dual energy X‐ray absorptiometry as a reference technique [77], taking into account advantages and disadvantages across available muscle mass measures. Therefore, data interpretation should always consider the principles behind each measurement technique before comparisons. One of the main limits, which neither dual energy X‐ray absorptiometry nor other adopted methods as CT scans, bioimpedance analysis and ultrasound evaluations, are able to detect fatty infiltrations within muscles, which is a common process caused by aging [77], which could lead to overestimation of muscle mass [78] in elderly or obese people.
Other aspects which needs to be considered are the relatively short intervention duration (mean 16 weeks) in the majority of studies, and study designs, since only three studies were randomized control trials while the majority were case series, therefore leading to a general low level of evidence. In addition, despite the single study results seem to be not homogeneous, no substantial differences in intervention length and assessment methods were present. Therefore, the moderator analysis performed could not identify a minimum dose–response to RT.
Conclusion
Based on the data acquired through our systematic literature search, RT protocols are able to moderately increase muscle mass in post-menopausal and elderly woman but not to reduce fat mass. Exercise frequency, number of exercises per training session, protocol length and age of the participants do not seem to significantly moderate the evaluated effects. Research needs to concentrate on defining training parameters as volume and intensity in order to help sport professionals to more effectively program RT protocols which would in turn counteract the effects of sarcopenia.
References
William J. Kraemer, Steven J. Fleck, Michael R. Deschenes. Exercise Physiology: Integrating Theory and Application 2015 2nd edition Lippincott Williams & Wilkins ISBN-10:145119319X.
Kraemer WJ, Nindl BC, Ratamess NA et al (2004) Changes in muscle hypertrophy in women with periodized resistance training. Med Sci Sports Exerc 36:697–708
Garber CE, Blissmer B, Deschenes MR et al (2011) American College of Sports, American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 43:1334–1359
Bodine SC, Stitt TN, Gonzalez M et al (2001) Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3:1014–1019
Drummond MJ, Dreyer HC, Pennings B et al (2008) Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol (1985) 104:1452–1461
Ahtiainen JP, Walker S, Peltonen H et al (2016) Heterogeneity in resistance training-induced muscle strength and mass responses in men and women of different ages. Age (Dordr) 38:10
Moro T, Brightwell CR, Deer RR et al (2018) Muscle protein anabolic resistance to essential amino acids does not occur in healthy older adults before or after resistance exercise training. J Nutr 148:900–909
Paoli A, Pacelli QF, Cancellara P et al (2016) Protein supplementation does not further increase Latissimus dorsi muscle fiber hypertrophy after eight weeks of resistance training in novice subjects, but partially counteracts the fast-to-slow muscle fiber transition. Nutrients 8:331
Hakkinen K, Pakarinen A, Kraemer WJ et al (2001) Selective muscle hypertrophy, changes in EMG and force, and serum hormones during strength training in older women. J Appl Physiol 91:569–580
Volpi E, Nazemi R, Fujita S (2004) Muscle tissue changes with aging. Curr Opin Clin Nutr Metab Care 7:405–410
Keller K, Engelhardt M (2014) Strength and muscle mass loss with aging process. Age and strength loss. Muscles Ligaments Tendons J 3:346–350
Zachwieja JJ, Yarasheski KE (1999) Does growth hormone therapy in conjunction with resistance exercise increase muscle force production and muscle mass in men and women aged 60 years or older? Phys Ther 79:76–82
Walston J, Fried LP (1999) Frailty and the older man. Med Clin North Am 83:1173–1194
Nevitt MC, Cummings SR, Kidd S et al (1989) Risk factors for recurrent nonsyncopal falls. A prospective study. JAMA 261:2663–2668
Dargent-Molina P, Favier F, Grandjean H et al (1996) Fall-related factors and risk of hip fracture: the EPIDOS prospective study. Lancet 348:145–149
Battaglia G, Giustino V, Messina G et al (2020) Walking in natural environments as geriatrician’s recommendation for fall prevention: preliminary outcomes from the “passiata day” model. Sustainability 12:2684
Janssen I, Baumgartner RN, Ross R et al (2004) Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol 159:413–421
Reid KF, Naumova EN, Carabello RJ et al (2008) Lower extremity muscle mass predicts functional performance in mobility-limited elders. J Nutr Health Aging 12:493–498
Castillo EM, Goodman-Gruen D, Kritz-Silverstein D et al (2003) Sarcopenia in elderly men and women: the Rancho Bernardo study. Am J Prev Med 25:226–231
Kalyani RR, Corriere M, Ferrucci L (2014) Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol 2:819–829
Messier V, Rabasa-Lhoret R, Barbat-Artigas S et al (2011) Menopause and sarcopenia: a potential role for sex hormones. Maturitas 68:331–336
Anagnostis P, Dimopoulou C, Karras S et al (2015) Sarcopenia in post-menopausal women: Is there any role for vitamin D? Maturitas 82:56–64
Sjoblom S, Suuronen J, Rikkonen T et al (2013) Relationship between postmenopausal osteoporosis and the components of clinical sarcopenia. Maturitas 75:175–180
Dornemann TM, McMurray RG, Renner JB et al (1997) Effects of high-intensity resistance exercise on bone mineral density and muscle strength of 40–50-year-old women. J Sports Med Phys Fit 37:246–251
Rhodes EC, Martin AD, Taunton JE et al (2000) Effects of one year of resistance training on the relation between muscular strength and bone density in elderly women. Br J Sports Med 34:18–22
Taaffe DR, Pruitt L, Pyka G et al (1996) Comparative effects of high- and low-intensity resistance training on thigh muscle strength, fiber area, and tissue composition in elderly women. Clin Physiol 16:381–392
Elliott KJ, Sale C, Cable NT (2002) Effects of resistance training and detraining on muscle strength and blood lipid profiles in postmenopausal women. Br J Sports Med 36:340–344
Delavier F, Gundill M (2015) Delavier’s women’s strength training anatomy workouts. Human Kinetics, Champaign, IL
Moher D, Liberati A, Tetzlaff J et al (2009) The preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097
Downs SH, Black N (1998) The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Commun Health 52:377–384
Cannon J, Kay D, Tarpenning KM et al (2007) Comparative effects of resistance training on peak isometric torque, muscle hypertrophy, voluntary activation and surface EMG between young and elderly women. Clin Physiol Funct Imaging 27:91–100
Correa CS, Cunha G, Marques N et al (2016) Effects of strength training, detraining and retraining in muscle strength, hypertrophy and functional tasks in older female adults. Clin Physiol Funct Imaging 36:306–310. https://doi.org/10.1111/cpf.12230
Cunha PM, Nunes JP, Tomeleri CM, et al (2020) Resistance training performed with single and multiple sets induces similar improvements in muscular strength, muscle mass, muscle quality, and IGF-1 in older women: a randomized controlled trial. J Strength Cond Res 34:1008–1016. https://doi.org/10.1519/JSC.0000000000002847
Nascimento MAD, Gerage AM, Silva D et al (2018) Effect of resistance training with different frequencies and subsequent detraining on muscle mass and appendicular lean soft tissue, IGF-1, and testosterone in older women. Eur J Sport Sci 19:199–207
Orsatti FL, Nahas EA, Maesta N et al (2008) Plasma hormones, muscle mass and strength in resistance-trained postmenopausal women. Maturitas 59:394–404
Orsatti FL, Nahas EA, Orsatti CL et al (2012) Muscle mass gain after resistance training is inversely correlated with trunk adiposity gain in postmenopausal women. J Strength Cond Res 26:2130–2139
Radaelli R, Wilhelm EN, Botton CE et al (2014) Effects of single vs. multiple-set short-term strength training in elderly women. Age (Dordr) 36(6):9720. https://doi.org/10.1007/s11357-014-9720-6
Ribeiro AS, Schoenfeld BJ, Fleck SJ et al (2017) Effects of traditional and pyramidal resistance training systems on muscular strength muscle mass, and hormonal responses in older women: a randomized crossover trial. J Strength Cond Res 31:1888–1896. https://doi.org/10.1519/JSC.0000000000001653
Santos L, Ribeiro AS, Schoenfeld BJ et al (2017) The improvement in walking speed induced by resistance training is associated with increased muscular strength but not skeletal muscle mass in older women. Eur J Sport Sci 17:488–494
Thiebaud RS, Loenneke JP, Fahs CA et al (2013) The effects of elastic band resistance training combined with blood flow restriction on strength, total bone-free lean body mass and muscle thickness in postmenopausal women. Clin Physiol Funct Imaging 33:344–352. https://doi.org/10.1111/cpf.12033
Botero JP, Shiguemoto GE, Prestes J et al (2013) Effects of long-term periodized resistance training on body composition, leptin, resistin and muscle strength in elderly post-menopausal women. J Sports Med Phys Fit 53:289–294
de Oliveira Júnior GN, de Sousa JFR, Carneiro M et al (2020) Resistance training-induced improvement in exercise tolerance is not dependent on muscle mass gain in post-menopausal women. Eur J Sport Sci. https://doi.org/10.1080/17461391.2020.1798511
Dos Santos L, Cyrino ES, Antunes M et al (2016) Changes in phase angle and body composition induced by resistance training in older women. Eur J Clin Nutr 70:1408–1413
Pina FLC, Nunes JP, Nascimento MA et al (2019) Similar effects of 24 weeks of resistance training performed with different frequencies on muscle strength, muscle mass, and muscle quality in older women. Int J Exerc Sci 12:623–635
Pina FLC, Nunes JP, Schoenfeld BJ et al (2020) Effects of different weekly sets-equated resistance training frequencies on muscular strength muscle mass, and body fat in older women. J Strength Cond Res 34:2990–2995
Tomeleri CM, Ribeiro AS, Nunes JP et al (2019) Influence of resistance training exercise order on muscle strength, hypertrophy, and anabolic hormones in older women: a randomized controlled trial. J Strength Cond Res. 34(11):3103-3109. https://doi.org/10.1519/JSC.0000000000003147
Vieira DCL, Nascimento DC, Tajra V et al (2020) High supervised resistance training in elderly women: the role of supervision ratio. Int J Exerc Sci 13:597–606
Dib MM, Tomeleri CM, Nunes JP et al (2020) Effects of three resistance exercise orders on muscular function and body composition in older women. Int J Sports Med 41:1024–1031
Janzen CL, Chilibeck PD, Davison KS (2006) The effect of unilateral and bilateral strength training on the bilateral deficit and lean tissue mass in post-menopausal women. Eur J Appl Physiol 97:253–260. https://doi.org/10.1007/s00421-006-0165-1
Churchward-Venne TA, Tieland M, Verdijk LB et al (2015) There are no nonresponders to resistance-type exercise training in older men and women. J Am Med Dir Assoc 16:400–411
Coelho-Júnior HJ, de Oliveiraonçalvez I, Sampaio RAC et al (2019) Periodized and non-periodized resistance training programs on body composition and physical function of older women. Exp Gerontol 121:10–18. https://doi.org/10.1016/j.exger.2019.03.001
Leenders M, Verdijk LB, van der Hoeven L et al (2013) Elderly men and women benefit equally from prolonged resistance-type exercise training. J Gerontol A Biol Sci Med Sci 68:769–779
Nunes JP, Ribeiro AS, Silva AM et al (2019) Improvements in phase angle are related with muscle quality index after resistance training in older women. J Aging Phys Act 27:515–520
Rabelo HT, Bezerra LA, Terra DF et al (2011) Effects of 24 weeks of progressive resistance training on knee extensors peak torque and fat-free mass in older women. J Strength Cond Res 25:2298–2303
Gambassi BB, Rodrigues B, Feriani DJ et al (2016) Effects of resistance training of moderate intensity on heart rate variability, body composition, and muscle strength in healthy elderly women. Sport Sci Health 12:389–395
Tremblay MS, LeBlanc AG, Kho ME et al (2011) Systematic review of sedentary behaviour and health indicators in school-aged children and youth. Int J Behav Nutr Phys Act 8:98
Dawson B, Trapp RG (2004) Basic and clinical biostatistics 4/E (ebook). McGraw-Hill Education, New York
Schoenfeld BJ, Grgic J, Ogborn D et al (2017) Strength and hypertrophy adaptations between low- vs high-load resistance training: a systematic review and meta-analysis. J Strength Cond Res 31:3508–3523
Grgic J, Schoenfeld BJ, Davies TB et al (2018) Effect of resistance training frequency on gains in muscular strength: a systematic review and meta-analysis. Sports Med 48:1207–1220
Borde R, Hortobágyi T, Granacher U (2015) Dose–response relationships of resistance training in healthy old adults: a systematic review and meta-analysis. Sports Med 45:1693–1720
Loenneke JP, Buckner SL, Dankel SJ et al (2019) Exercise-induced changes in muscle size do not contribute to exercise-induced changes in muscle strength. Sports Med 49:987–991
Gabriel DA, Kamen G, Frost G (2006) Neural adaptations to resistive exercise: mechanisms and recommendations for training practices. Sports Med 36:133–149
Schoenfeld BJ (2010) The mechanisms of muscle hypertrophy and their application to resistance training. J Strength Cond Res 24:2857–2872
Endo Y, Nourmahnad A, Sinha I (2020) Optimizing skeletal muscle anabolic response to resistance training in aging. Front Physiol 11:874–874
Foster-Burns SB (1999) Sarcopenia and decreased muscle strength in the elderly woman: resistance training as a safe and effective intervention. J Women Aging 11:75–85
Leite RD, Prestes J, Pereira GB et al (2010) Menopause: highlighting the effects of resistance training. Int J Sports Med 31:761–767
Shaw BS, Gouveia M, McIntyre S et al (2016) Anthropometric and cardiovascular responses to hypertrophic resistance training in postmenopausal women. Menopause 23:1176–1181
Morton RW, Murphy KT, McKellar SR et al (2018) A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med 52:376–384
Paoli A, Canato M, Toniolo L et al (2011) The ketogenic diet: an underappreciated therapeutic option? Clin Terapeutica 162:e145–e153
Nelson ME, Fiatarone MA, Morganti CM et al (1994) Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures. A randomized controlled trial. JAMA 272:1909–1914
Larsson L (1983) Histochemical characteristics of human skeletal muscle during aging. Acta Physiol Scand 117:469–471
Hida T, Shimokata H, Sakai Y et al (2016) Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J 25:3424–3431
Di Monaco M, Vallero F, Di Monaco R et al (2011) Prevalence of sarcopenia and its association with osteoporosis in 313 older women following a hip fracture. Arch Gerontol Geriatr 52:71–74
Woodward MJ, Lu CW, Levandowski R et al (2015) The exercise prescription for enhancing overall health of midlife and older women. Maturitas 82:65–71
Lemos T, Gallagher D (2017) Current body composition measurement techniques. Curr Opin Endocrinol Diabetes Obes 24:310–314
Buehring B, Siglinsky E, Krueger D et al (2018) Comparison of muscle/lean mass measurement methods: correlation with functional and biochemical testing. Osteoporos Int 29:675–683
Buckinx F, Landi F, Cesari M et al (2018) Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle
Segal NA, Glass NA, Baker JL et al (2009) Correcting for fat mass improves DXA quantification of quadriceps specific strength in obese adults aged 50–59 years. J Clin Densitom 12:299–305
Funding
Open access funding provided by Università degli Studi di Padova within the CRUI-CARE Agreement. The authors did not receive any specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest relevant to the content of this review.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thomas, E., Gentile, A., Lakicevic, N. et al. The effect of resistance training programs on lean body mass in postmenopausal and elderly women: a meta-analysis of observational studies. Aging Clin Exp Res 33, 2941–2952 (2021). https://doi.org/10.1007/s40520-021-01853-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-021-01853-8