Abstract
Purpose of Review
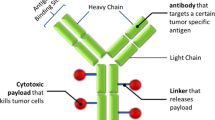
This article provides a brief overview of bispecific antibody (BsAb) mechanisms of action, structures, and pharmacokinetic (PK) properties, including absorption, distribution, and elimination. Recent trend in BsAb development is also introduced from a PK perspective.
Recent Findings
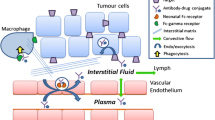
Advances in biotechnology have led to the development of diverse BsAb formats, which provide a wide range of options to tailor the design of BsAbs to match the proposed mechanisms of action and to optimize the intended therapies. Understanding the PK properties of BsAbs and the important factors influencing PK is critical for the rationale design and development of BsAb therapeutics. PK behavior of a BsAb is dependent on its format and could be governed by molecular weight, physicochemical properties, interaction with Fc receptors, and binding to target, etc. Due to their multi-binding properties, BsAbs may have superior target tissue deliveries (e.g., brain, solid tumors) and additional unique PK properties compared to conventional mAbs, which may offer therapeutic advantages. Despite the many unknowns about BsAb disposition, it is generally believed that an ideal BsAb should have a balance between favorable systemic exposure (typically a long half-life is preferred) and appropriate distribution and tissue penetration.
Summary
BsAb has unique mechanisms of action, structures, and PK properties when compared to conventional monoclonal antibody (mAb) and also shares similar pathways that contribute to PK characteristics of conventional mAb. Understanding the PK of BsAbs and the important factors influencing the PK is critical for the rationale design and development of BsAb therapeutics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Papers of particular interest, published recently, have been highlighted as: • Of importance ••Of major importance
•• Spiess C, Zhai Q, Carter PJ. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol. 2015;67(2 Pt A):95–106. This article provides a comprehensive review of bi-specific antibody molecular formats, mechanisms of action, preclincal and clinical development
Kontermann RE, Brinkmann U. Bispecific antibodies. Drug Discov Today. 2015;20(7):838–47.
Thakur A, Lum LG. “NextGen” biologics: bispecific antibodies and emerging clinical results. Expert Opin Biol Ther. 2016;16(5):675–88.
Bano JC, Chames P, Baty D, Kerfelec B. Taking up cancer immunotherapy challenges: bispecific antibodies, the path forward? Antibodies. 2016; 5(1).
(EMA), E.M.A. Catumaxomab. Summary of product characteristics (SmPC). 2009; Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000972/WC500051809.pdf.
(EMA), E.M.A. Blinatumomab. Summary of product characteristics (SmPC).. 2016; Available from: https://ec.europa.eu/health/documents/community-register/2015/20151123133349/anx_133349_en.pdf.
(FDA), U.F.a.D.A. Blinatumomab. Package Insert.. 2014; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125557lbl.pdf.
Brinkmann U, Kontermann RE. The making of bispecific antibodies. MAbs. 2017;9(2):182–212.
Nisonoff A, Wissler FC, Lipman LN. Properties of the major component of a peptic digest of rabbit antibody. Science. 1960;132(3441):1770–1.
Webster CI, et al. Brain penetration, target engagement, and disposition of the blood-brain barrier-crossing bispecific antibody antagonist of metabotropic glutamate receptor type 1. FASEB J. 2016;30(5):1927–40.
Yu YJ, et al. Therapeutic bispecific antibodies cross the blood-brain barrier in nonhuman primates. Sci Transl Med. 2014;6(261):261ra154.
Couch JA, et al. Addressing safety liabilities of TfR bispecific antibodies that cross the blood-brain barrier. Sci Transl Med. 2013;5(183):183ra57. 1-12
Lutterbuese R, et al. T cell-engaging BiTE antibodies specific for EGFR potently eliminate KRAS- and BRAF-mutated colorectal cancer cells. Proc Natl Acad Sci U S A. 2010;107(28):12605–10.
Junttila TT, et al. Antitumor efficacy of a bispecific antibody that targets HER2 and activates T cells. Cancer Res. 2014;74(19):5561–71.
Svecova, D., et al. Safety and efficacy of multiple ascending doses of subcutaneous M1095, an anti-interleukin-17A/F bispecific nanobody, in patients with moderate-to-severe psoriasis. Available from: http://www.ablynx.com/uploads/data/files/170301_m1095_psoriasis_003%20for%20aad2017.pdf
Dong J, et al. A stable IgG-like bispecific antibody targeting the epidermal growth factor receptor and the type I insulin-like growth factor receptor demonstrates superior anti-tumor activity. MAbs. 2011;3(3):273–88.
Wec AZ, et al. A “Trojan horse” bispecific-antibody strategy for broad protection against ebolaviruses. Science. 2016;354(6310):350–4.
Sebastian M, et al. Catumaxomab: a bispecific trifunctional antibody. Drugs Today (Barc). 2009;45(8):589–97.
•• Ruf P, et al. Pharmacokinetics, immunogenicity and bioactivity of the therapeutic antibody catumaxomab intraperitoneally administered to cancer patients. Br J Clin Pharmacol. 2010;69(6):617–25. This article reported the results of clincial and pre-clinical pharmacokinetic studies of catumaxomab. Bioavailability of catumaxomab was found to be related to tumor burden and effector cells in the animal model
clinicaltrials.gov. A phase 1b open-label study investigating the safety and pharmacokinetics of administration of subcutaneous blinatumomab for the treatment of relapsed/refractory indolent non-Hodgkin’s lymphoma. Available from: https://clinicaltrials.gov/ct2/show/NCT02961881
Porter CJ, Charman SA. Lymphatic transport of proteins after subcutaneous administration. J Pharm Sci. 2000;89(3):297–310.
Richter WF, Bhansali SG, Morris ME. Mechanistic determinants of biotherapeutics absorption following SC administration. AAPS J. 2012;14(3):559–70.
Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci. 2004;93(11):2645–68.
Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84(5):548–58.
•• Zhu M, et al. Blinatumomab, a Bispecific T-cell Engager (BiTE((R))) for CD-19 targeted cancer immunotherapy: clinical pharmacology and its implications. Clin Pharmacokinet. 2016;55(10):1271–88. This article reported the PK/PD related aspects of blinatumomab from multiple clincial studies. Mechanism invovled in blinatumomab disposition, determinants of PK varibility, and PK/PD considerations in dose selection were also discussed
Baxter LT, et al. Physiologically based pharmacokinetic model for specific and nonspecific monoclonal antibodies and fragments in normal tissues and human tumor xenografts in nude mice. Cancer Res. 1994;54(6):1517–28.
Wurster U, Haas J. Passage of intravenous immunoglobulin and interaction with the CNS. J Neurol Neurosurg Psychiatry. 1994;57(Suppl):21–5.
Yu YJ, et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci Transl Med. 2011;3(84):84ra44.
van Osdol W, Fujimori K, Weinstein JN. An analysis of monoclonal antibody distribution in microscopic tumor nodules: consequences of a “binding site barrier”. Cancer Res. 1991;51(18):4776–84.
Adams GP, et al. High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules. Cancer Res. 2001;61(12):4750–5.
Verel I, et al. Tumor targeting properties of monoclonal antibodies with different affinity for target antigen CD44V6 in nude mice bearing head-and-neck cancer xenografts. Int J Cancer. 2002;99(3):396–402.
Rudnick SI, et al. Influence of affinity and antigen internalization on the uptake and penetration of anti-HER2 antibodies in solid tumors. Cancer Res. 2011;71(6):2250–9.
Bortoletto N, et al. Optimizing anti-CD3 affinity for effective T cell targeting against tumor cells. Eur J Immunol. 2002;32(11):3102–7.
List T, Neri D. Biodistribution studies with tumor-targeting bispecific antibodies reveal selective accumulation at the tumor site. MAbs. 2012;4(6):775–83.
Nelson AL. Antibody fragments: hope and hype. MAbs. 2010;2(1):77–83.
Sun LL, et al. Anti-CD20/CD3 T cell-dependent bispecific antibody for the treatment of B cell malignancies. Sci Transl Med. 2015;7(287):287ra70.
Rothe A, et al. A phase 1 study of the bispecific anti-CD30/CD16A antibody construct AFM13 in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2015;125(26):4024–31.
• Deng R, et al. Monoclonal antibodies: what are the pharmacokinetic and pharmacodynamic considerations for drug development? Expert Opin Drug Metab Toxicol. 2012;8(2):141–60. This article is a recent comprehensive review paper on conventional antibody’s PK and PD
Yazaki PJ, et al. A series of anti-CEA/anti-DOTA bispecific antibody formats evaluated for pre-targeting: comparison of tumor uptake and blood clearance. Protein Eng Des Sel. 2013;26(3):187–93.
Datta-Mannan A, et al. Aberrant bispecific antibody pharmacokinetics linked to liver sinusoidal endothelium clearance mechanism in cynomolgus monkeys. MAbs. 2016;8(5):969–82.
Croasdale R, et al. Development of tetravalent IgG1 dual targeting IGF-1R-EGFR antibodies with potent tumor inhibition. Arch Biochem Biophys. 2012;526(2):206–18.
Dong J, et al. Stable IgG-like bispecific antibodies directed toward the type I insulin-like growth factor receptor demonstrate enhanced ligand blockade and anti-tumor activity. J Biol Chem. 2011;286(6):4703–17.
Hu L, Hansen RJ. Issues, challenges, and opportunities in model-based drug development for monoclonal antibodies. J Pharm Sci. 2013;102(9):2898–908.
Li L, Gardner I, Gill K, Modeling the binding kinetics of bispecific antibodies under the framework of a minimal human PBPK model, in AAPS NBC2014.
Gadkar K, et al. Quantitative systems pharmacology: a promising approach for translational pharmacology. Drug Discov Today Technol. 2016;21-22:57–65.
Chudasama VL, et al. Simulations of site-specific target-mediated pharmacokinetic models for guiding the development of bispecific antibodies. J Pharmacokinet Pharmacodyn. 2015;42(1):1–18.
Gadkar K, et al. Mathematical PKPD and safety model of bispecific TfR/BACE1 antibodies for the optimization of antibody uptake in brain. Eur J Pharm Biopharm. 2016;101:53–61.
Acknowledgements
The authors thank Tommy Li and Xin Miao for editorial contribution and helpful discussion.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors received no financial support in the writing of this manuscript. Both authors are employees of Janssen Research & Development, LLC. The opinions expressed in this publication are those of the authors and do not necessarily reflect those of the company who employs them.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Molecular Drug Disposition
Rights and permissions
About this article
Cite this article
Chen, Y., Xu, Y. Pharmacokinetics of Bispecific Antibody. Curr Pharmacol Rep 3, 126–137 (2017). https://doi.org/10.1007/s40495-017-0090-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40495-017-0090-5