Abstract
Introduction
First-line treatment of persistent, recurrent, or metastatic (advanced) cervical cancer in patients who have a combined positive score (CPS) ≥ 1 with pembrolizumab + chemotherapy versus standard-of-care chemotherapy provides meaningful improvements in overall survival. We conducted a cost-effectiveness analysis from a US payer perspective. A societal perspective scenario was also considered, including productivity gains.
Methods
The cost-effectiveness of pembrolizumab + chemotherapy versus chemotherapy was assessed using a state-transition model comprising the health states “pre-progression,” “post-progression,” and “death,” with a 1-week cycle length and 50-year time horizon. Patient-level KEYNOTE-826 data informed the efficacy, safety, and health-related quality of life of pembrolizumab + chemotherapy versus chemotherapy at first-line and subsequent treatments. Real-world data were sought to cost subsequent treatments according to US clinical practice. Transition probabilities were derived from parametric survival models fit to time-to-progression, progression-free survival, and post-progression survival patient-level KEYNOTE-826 data. Sensitivity analyses explored the impact on outcomes from variables such as bevacizumab use.
Results
According to the state-transition model, pembrolizumab + chemotherapy extended mean life expectancy versus chemotherapy from 1.8 to 6.7 life-years. The mean gain of 4.9 life-years/patient was mostly caused by pembrolizumab delaying progression. Total discounted quality-adjusted life-years (QALY) were 5.0 and 1.3 per patient for pembrolizumab + chemotherapy and chemotherapy, respectively (mean gain: 3.7 QALY/patient). Pembrolizumab + chemotherapy had comparable safety outcomes to chemotherapy alone. Total costs incurred were US $320,247 (pembrolizumab + chemotherapy) versus US $105,446 (chemotherapy; mean incremental costs: US $214,801/patient). The incremental cost-effectiveness ratio of pembrolizumab + chemotherapy versus chemotherapy was US $58,446/QALY. Sensitivity analyses showed results were insensitive to bevacizumab use. Including productivity gains led to an incremental cost-effectiveness ratio of US $58,385 per QALY.
Conclusions
Our model-based analysis suggests that first-line treatment of pembrolizumab + chemotherapy in advanced cervical cancer with a CPS ≥ 1 offers a substantial clinical benefit over standard-of-care chemotherapy alone and is cost-effective at a willingness-to-pay threshold of US $150,000. The approximate doubling of life-years and QALYs associated with pembrolizumab + chemotherapy represents a step improvement in the treatment of advanced cervical cancer.
Trial Registration
ClinicalTrials.gov Identification Number: NCT03635567.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Women with persistent, recurrent, or metastatic cervical cancer have a poor prognosis and an unmet need for life-extending treatment options. |
A study based on an interim analysis of the phase III KEYNOTE-826 trial previously demonstrated that pembrolizumab + chemotherapy provided a cost-effective alternative to standard-of-care chemotherapy alone for the treatment of advanced cervical cancer. |
This study further explores the cost-effectiveness of pembrolizumab + chemotherapy using data from the final analysis of KEYNOTE-826, which showed a prolonged plateau in progression-free survival for pembrolizumab + chemotherapy-treated patients, and considers a societal perspective in the United States (US). |
What was learned from the study? |
Pembrolizumab + chemotherapy provides a twofold increase in life-years and quality-adjusted life-years (QALYs) in people with advanced cervical cancer compared with standard-of-care chemotherapy. |
Pembrolizumab + chemotherapy was shown to be cost-effective at a willingness-to-pay threshold of US $150,000/QALY gained compared with standard-of-care chemotherapy, and including productivity gains led to an incremental cost-effectiveness ratio of US $58,385 per QALY. |
The clinical and cost-effectiveness results from this study can be used to assist US payer decision-making on the use of pembrolizumab + chemotherapy for the treatment of advanced cervical cancer. |
Introduction
Cervical cancer is the fourth most common cancer in women [1]. In the United States, there will be an estimated 13,820 cases of cervical cancer in 2024, which may result in 4360 deaths [2]. Despite screening in the United States, it remains the second leading cause of cancer death in women aged 20–39 years [3].
Cervical cancer is characterized as advanced if it is persistent, recurrent, or metastatic. Symptoms at an advanced stage include fatigue, feeling unwell, weight loss, gripping abdominal pain, blood in urine, and excessive vomiting. Besides physical symptoms, worries about premature death increase anxiety and depression in young women with advanced cervical cancer [4].
At an advanced stage where cervical cancer has metastasized, the 5-year survival rate is 19% [2] and treatment options are limited. Around 15% of cases are diagnosed when advanced [2], highlighting the unmet need for effective treatments for patients with advanced cervical cancer.
In October 2021, the US Food and Drug Administration (FDA) approved KEYTRUDA® (pembrolizumab), in combination with chemotherapy, as a first-line treatment for patients with advanced cervical cancer who have a programmed death-ligand 1 (PD-L1)-combined positive score ≥ 1 as determined by an FDA-approved test, based on KEYNOTE-826 trial results (NCT03635567) [5].
The interim analysis of KEYNOTE-826 (May 2021 data cutoff) demonstrated that pembrolizumab + standard-of-care chemotherapy (with or without bevacizumab) provided statistically significant overall and progression-free survival improvements in patients with advanced cervical cancer [6]. In the final analysis (October 2022 data cutoff), median overall survival in the pembrolizumab group was 28.6 months versus 16.5 months for chemotherapy [7]. Incidence of adverse events was similar across arms and consistent with the known safety profiles of pembrolizumab and chemotherapy and the KEYNOTE-826 trial indication [6, 7].
Based on KEYNOTE-826 results, the National Comprehensive Cancer Network added pembrolizumab combination therapy to the list of preferred regimens in its 2022 guidelines as first-line therapy for the treatment of PD-L1-positive cervical cancer in the United States. Pembrolizumab is distinct from standard chemotherapies (cisplatin/carboplatin + paclitaxel ± bevacizumab) as it represents the only first-line immuno-oncology agent approved by the FDA to treat advanced cervical cancer [8].
A cost-effectiveness study using patient-level data from an interim analysis of KEYNOTE-826 demonstrated that pembrolizumab + chemotherapy provided a cost-effective alternative to chemotherapy alone [9]. Here we use the final analysis of KEYNOTE-826 to further explore the cost-effectiveness of pembrolizumab + chemotherapy.
Methods
This study was based on previously collected, anonymized clinical trial data from KEYNOTE-826 provided by the study sponsor; as such, it was exempt from institutional review board or ethical approval. The KEYNOTE-826 trial was approved by the appropriate ethics body at each participating center [6, 7].
We built a cost-effectiveness model in Microsoft Excel with a 1-week cycle length and 50-year time horizon. The model captures lifetime effects and costs, given an average age of 51.0 years at diagnosis. The cycle length allowed accurate accounting for the dosing schedules of included interventions and avoided material half-cycle correction. We adopted a US healthcare perspective and followed relevant guidelines, including the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 statement [10,11,12,13].
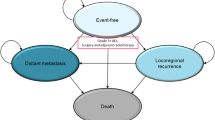
In KEYNOTE-826, progression-free survival was seen to plateau for patients receiving pembrolizumab + chemotherapy. This resulted in scenarios where the extrapolations of progression-free survival and overall survival crossed in the partitioned survival model, as this approach includes no structural link between these endpoints. As the model structure was anticipated to create uncertainty around the results, we followed best practice and developed both a state-transition and partitioned survival analysis approach [14]. We used the state-transition approach in our base case, leveraging KEYNOTE-826 individual patient-level data to derive time-to-progression and post-progression survival estimates to inform transition, alongside progression-free and overall survival (Fig. 1). The partitioned survival approach is considered to quantify structural uncertainty [15].
Three-health state semi-Markov state-transition model. Numbers in square brackets indicate transition probabilities, defined in the figure. PFS(t, arm), TTP(t, arm), and PPS(t, arm) are the survivor functions for progression-free survival, time to progression, and post-progression survival, respectively, at time t and the given treatment arm. PFS progression-free survival, PPS post-progression survival, TTP time to progression
Decision Problem
The population modeled was patients with advanced cervical cancer and a combined positive score ≥ 1 [16]. This population accounted for 89% of the patients in KEYNOTE-826 [6]. KEYNOTE-826 was designed to evaluate pembrolizumab + chemotherapy (paclitaxel + cisplatin or paclitaxel + carboplatin) ± bevacizumab versus chemotherapy ± bevacizumab in women with cervical cancer [6].
Within the model, dosing for pembrolizumab + chemotherapy is aligned with KEYNOTE-826 and the FDA license [16]. Pembrolizumab is given until progression for up to 2 years or 35 treatment cycles; hence, a 105-week stopping rule was applied. Except for bevacizumab, which has no stopping rule, chemotherapy in both arms is given until progression for a maximum of six treatment cycles; hence, an 18-week stopping rule was applied for the concerned regimens.
Subsequent treatment was modeled as per KEYNOTE-826: 48% of patients who progressed in KEYNOTE-826 received second-line chemotherapy, which aligns with US real-world data [3]. For simplification, the model only included second-line treatment costs administered to ≥ 3% of patients in either arm of KEYNOTE-826.
Cost-effectiveness was assessed in terms of costs per life-year and quality-adjusted life-year (QALY) gained. Consistent with the current Institute for Clinical and Economic Review Value Assessment Framework, a US $150,000/QALY willingness-to-pay threshold was used [10].
Model Inputs
Efficacy
The median follow-up period in KEYNOTE-826 was 28.6 months; therefore, it was necessary to perform extrapolations to estimate long-term outcomes for a lifetime time horizon of up to 50 years. Standard parametric survival models (exponential, Weibull, log-normal, log-logistic, Gompertz, and generalized gamma) were fit to the full individual patient-level data separately for pembrolizumab + chemotherapy and chemotherapy (Supplementary Material 1). More flexible methods were explored if one-piece models did not provide a reasonable fit to the patient-level data. These methods included “two-piece” models with the Kaplan–Meier curve followed by a parametric survival model fit to the data from a certain time point onwards, and Royston–Parmar spline models with up to three knots [17]. Curve selection was based on visual fit to patient-level data, the clinical plausibility of long-term extrapolations and hazard functions, and the statistical fit to the patient-level data. Data from the Gynecologic Oncology Group (GOG)-240 trial were used to validate extrapolations [18].
Time-to-Event Modeling
Time-to-progression and progression-free and post-progression survival inputs were informed by data from the primary KEYNOTE-826 analysis. In the base case, three-knot spline models were used for time to progression and progression-free survival. Deaths were recorded as an event for progression-free survival but as a censoring event for time to progression. Therefore, progression-free survival is the time to a composite endpoint of progression or death, whereas time to progression is based on disease progression alone. The flexible spline model follows the change in hazard, meaning it captured the plateau in pembrolizumab patient survival well. The model assumptions and selection process are described in Supplementary Materials 1 and 2, respectively.
As the number of deaths in KEYNOTE-826 before progression was small, pre-progression mortality was estimated from time to progression and progression-free survival using the formula
where Sx(t) is the survival function for endpoint(x) at time(t).
To avoid clinically implausible pre-progression mortality estimates in the cost-effectiveness model, the same modeling approach and specification were selected for progression-free survival as for time to progression (Fig. 2).
In the base-case analysis, post-progression survival data from KEYNOTE-826 were extrapolated using generalized gamma models fit to all Kaplan–Meier data (Supplementary Material 1).
When using the partitioned survival model approach, progression-free survival was modeled using the same three-knot spline as the state-transition model base case. Similarly, overall survival was modeled using three-knot spline models.
Safety
In the base case, we included grade ≥ 3 drug-related adverse events that occurred in ≥ 5% of patients in either arm, including anemia, neutropenia, urinary tract infection, hypertension, thrombocytopenia, febrile neutropenia, and decreased platelet, white blood cell, and neutrophil counts. The mean duration of adverse events was obtained from KEYNOTE-826.
Health-Related Quality of Life
The European Quality of Life 5-Dimension 5-Level version instrument was used to measure generic health status in patients enrolled in KEYNOTE-826. We used patient-level data from KEYNOTE-826 and the US-specific value set to calculate utilities [19].
We computed utilities by time to death to capture how pembrolizumab impacts quality of life (QoL) in patients with advanced cervical cancer. Time-to-death utilities were deemed more appropriate than utility inputs stratified by progression status because “pseudoprogression” issues can be encountered with immuno-oncology drugs, where the action of treatment may be mistaken for disease progression. They also provide finer gradations in utility across patients compared with progression-based utilities [20]. In the base case, we applied a regression model with time-to-death categories and grade ≥ 3 adverse events to predict treatment-independent utilities for use in the analysis (Supplementary Material 1). Utilities in the model were capped using US-specific data on general population utility [21].
Costs
The analysis considered drug acquisition (wholesale acquisition cost) [22], treatment administration, PD-L1 marker testing, adverse events, resource use, and end-of-life costs. Costs were adjusted to 2022 US dollars using the US Bureau of Labor Statistics, Consumer Price Index, and Medical Care inflation calculator. Costs and QALYs were discounted at 3% per year.
Time on treatment was based on KEYNOTE-826 patient-level data. Extrapolation was not needed for pembrolizumab, paclitaxel, cisplatin, or carboplatin, as the clinical trial covered the maximum duration these treatments were given in both arms. For bevacizumab, time-on-treatment patient-level data were extrapolated using a two-piece log-logistic model that was fit from 46 weeks onward (Supplementary Material 1). We sourced wholesale acquisition cost prices from the AnalySource database [22]. Treatment administration, PD-L1 testing, and other resource use costs were sourced from the Centers for Medicare and Medicaid Services [22, 23]. The cost of PD-L1 testing reflected the cost per PD-L1-positive patient identified rather than the cost per test, with an average of 1.1 tests required per positive patient identified. Clinical experts informed resource use frequencies in the pre- and post-progression health states, assuming no differences between treatment arms. Adverse events were costed using Medicare severity diagnosis-related groups [10]. We retrieved end-of-life costs from the literature [24]. The tested scenarios, model assumptions, and inputs are reported in Supplementary Material 1.
Sensitivity Analyses
We performed one-way sensitivity analyses to explore the impact of uncertainty in individuals’ input parameters on results and a probabilistic sensitivity analysis of 5000 iterations to explore the combined uncertainty of all inputs on results.
Scenario Analyses
Multiple scenario analyses were conducted to investigate various modeling assumptions (Supplementary Material 3).
A key scenario was assessing the structural uncertainty introduced by the choice of modeling approach—state-transition or partitioned survival. The partitioned survival model was parameterized using the same inputs as the state-transition model in every plausible instance. The only difference was modeling survival using an extrapolation of overall survival data from KEYNOTE-826. Overall survival was extrapolated using a Royston–Parmar spline model that had three knots.
Treatment options in second line are rapidly evolving, and real-world data suggest that tisotumab vedotin and pembrolizumab are widely administered at second line in this US subpopulation [25]. Therefore, we performed a scenario analysis where tisotumab vedotin and pembrolizumab were added to subsequent treatment options. Inputs for this scenario were clinically informed and resulted in the following assumptions: in the pembrolizumab arm, 60% of progressed patients receive tisotumab vedotin, 20% pembrolizumab, and 20% chemotherapy. In second-line chemotherapy, we assumed that 75% received tisotumab vedotin and 25% pembrolizumab. We evaluated results separately in patients who received bevacizumab and those who did not using a 90-day friction-cost method [26].
We also explored pembrolizumab’s societal impact by including productivity gains. These were calculated using inputs from Supplementary Material 1 and assume that patients with progressed disease no longer work, while those who are progression-free work part-time.
Results
Pembrolizumab extended the mean life expectancy of patients with advanced cervical cancer from 1.8 to 6.7 life-years (mean life-years gained/patient: 4.9). The gain in life expectancy was mostly attributable to delayed disease progression as a result of pembrolizumab treatment; hence, in most life-years gained, patients have a favorable health-related QoL. Total QALYs were 5.0 and 1.3/patient for pembrolizumab + chemotherapy and chemotherapy, respectively (mean discounted QALYs gained/patient: 3.7) (Table 1). The discounted costs incurred with pembrolizumab + chemotherapy were US $320,247 versus US $105,446 with chemotherapy (mean incremental costs/patient: US $214,801). The difference in costs between pembrolizumab + chemotherapy and chemotherapy was mainly driven by pembrolizumab acquisition costs. The incremental cost-effectiveness ratio for pembrolizumab + chemotherapy versus chemotherapy was US $44,037/life-year and US $58,446/QALY.
Probabilistic sensitivity analysis results are presented in Fig. 3. The probabilistic incremental cost-effectiveness ratios of US $43,395/life-year and US $57,597/QALY align with deterministic incremental cost-effectiveness ratios. The cost-effectiveness acceptability curve shows that, at willingness-to-pay thresholds of US $50,000, US $100,000, and US $150,000 per QALY, pembrolizumab + chemotherapy was cost-effective in 14.9%, 99.5%, and 99.6% of the iterations, respectively. The one-way sensitivity analyses represented in Fig. 4 demonstrate that the cost-effectiveness of pembrolizumab + chemotherapy is relatively robust to uncertainty in model inputs.
One-way sensitivity and scenario analysis results. S represents the scenarios resulting in significant changes in the ICER. bev bevacizumab, BICR blinded independent central review, ICER incremental cost-effectiveness ratio, PEM pembrolizumab, PFS progression-free survival, pSoC pembrolizumab + standard-of-care chemotherapy, QALY quality-adjusted life-year, OWSA one-way sensitivity analysis, TTP time to progression, TV tisotumab vedotin
The scenario analysis where pembrolizumab and tisotumab vedotin were included at second line resulted in a slightly less favorable incremental cost-effectiveness ratio (US $60,783/QALY). The scenario exploring productivity gains due to treatment with pembrolizumab + chemotherapy led to a similar incremental cost-effectiveness ratio (US $58,385/QALY).
Scenarios and deterministic sensitivity analysis exploring bevacizumab use suggested that, although costs and outcomes differed between patients who had or had not received bevacizumab, incremental cost-effectiveness results between pembrolizumab strategies were very similar and insensitive to bevacizumab use. This aligns with recent evidence demonstrating that pembrolizumab provides a meaningful benefit regardless of whether it is provided in combination with bevacizumab [27].
Model results are sensitive to the approach used (state-transition or partitioned survival), with the partitioned survival approach resulting in an incremental cost-effectiveness ratio of US $73,636/QALY. This increased the incremental cost-effectiveness ratio but, along with all the other scenarios, is still below the Institute for Clinical and Economic Review’s willingness-to-pay threshold of US $150,000/QALY [10].
Discussion
Summary of Main Results
This cost-effectiveness model has demonstrated that, over a lifetime, pembrolizumab results in an approximate doubling of expected life-years and QALYs per patient. The incremental cost-effectiveness ratio for pembrolizumab + chemotherapy versus chemotherapy was US $58,446/QALY, which is below the US $150,000 willingness-to-pay threshold proposed in the Institute for Clinical and Economic Review’s framework [10]. Decision uncertainty is also low, with each of the 1000 probabilistic iterations falling below this threshold.
The probabilistic sensitivity analysis and one-way sensitivity analyses demonstrate that the cost-effectiveness of pembrolizumab + chemotherapy is relatively robust to uncertainty in model inputs. The scenario analyses demonstrate that the base case incremental cost-effectiveness ratios may be conservative, with many scenarios producing more favorable incremental cost-effectiveness ratios.
Results in the Context of Published Literature
Our results differ from those reported by Shi et al. and Barrington et al. [28, 29]. Shi et al. report an incremental cost-effectiveness ratio of US $253,322/QALY, compared with the US $73,636/QALY in our analysis using the equivalent model structure. The difference in incremental cost-effectiveness ratios is mainly caused by differences in estimated life-years, and consequently, QALYs gained between the analyses. While we estimate QALYs gained to be 2.9 with the partitioned survival model approach, Shi et al. estimate 0.8 [29]. This is likely driven by the use of the final analysis for KEYNOTE-826 in our study, which further accentuates the plateau in progression-free survival for patients who received pembrolizumab + chemotherapy, whereas Shi et al. used data from published reports on the interim analysis. Barrington et al. report an incremental cost-effectiveness ratio of US $341,361/QALY but present an insufficient description of the analytical framework, survival inputs, and settings to clarify whether differences in the modeling approach drove the incremental cost-effectiveness ratio differences [28]. Moreover, Barrington et al. used KEYNOTE-826 median values for survival instead of established methods to estimate mean and long-term survival, which is likely to underestimate benefits for the pembrolizumab + chemotherapy group compared with chemotherapy alone. Neither study can be as robust as the one presented in this paper, as both relied on the literature for efficacy and utility estimates, whereas our study presents analyses using patient-level data from KEYNOTE-826.
Strengths and Weaknesses
By using KEYNOTE-826 patient-level data to derive model inputs, our study provides an accurate analysis of pembrolizumab + chemotherapy cost-effectiveness. Furthermore, we have demonstrated that the cost-effectiveness of pembrolizumab + chemotherapy is not dependent on the modeling approach employed. Also, we modeled subsequent treatments in line with US clinical practice and performed a scenario analysis to assess the impact of tisotumab vedotin and pembrolizumab at second line, relying on expert advice and external trial data to inform costs and post-progression survival [30,31,32]. Finally, we validated long-term extrapolations of efficacy with observed 4-year data from the GOG-240 study [18].
However, even with final analysis data the follow-up was incomplete, so some extrapolation was necessary. Also, some subsequent treatments received by patients in the trial did not align with real-world treatments.
Implications for Practice and Future Research
With current standard of care, the 5-year survival rate for women with advanced cervical cancer is 17% and treatment options are few. Adding pembrolizumab to first-line treatment extends the long-term survival of patients with advanced cervical cancer by delaying progression and improving QoL; its cost-effectiveness ratio lies below that considered cost-effective by the Institute for Clinical and Economic Review [10].
This is the first cost-effectiveness evaluation of pembrolizumab in advanced cervical cancer to consider indirect costs in a scenario analysis from a US payer perspective. This analysis shows that pembrolizumab offers substantial productivity gains—highlighting the importance of indirect costs in relatively young populations. Future research should aim to validate subsequent treatment proportions for tisotumab vedotin and pembrolizumab when more published data become available.
Conclusion
This evaluation demonstrates that pembrolizumab + chemotherapy is cost-effective for first-line treatment of patients in the United States who have advanced cervical cancer with a combined positive score ≥ 1, at a willingness-to-pay threshold of US $150,000/QALY.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available. Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data-sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request.
References
World Health Organization (WHO). Health topics: cervical cancer overview https://www.who.int/health-topics/cervical-cancer#tab=tab_1. Accessed June 10, 2024.
The Surveillance Epidemiology and End Results Program (SEER). Cancer stat facts: cervical cancer. https://seer.cancer.gov/statfacts/html/cervix.html. Accessed June 10, 2024.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33.
Tosic Golubovic S, Binic I, Krtinic D, et al. Risk factors and predictive value of depression and anxiety in cervical cancer patients. Medicina (Kaunas). 2022;58(4).
Food and Drug Administration (FDA). FDA approves pembrolizumab combination for the first-line treatment of cervical cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-combination-first-line-treatment-cervical-cancer#:~:text=On%20October%2013%2C2021%2C%20the,by%20an%20FDA%2Dapproved%20test. Accessed June 10, 2024.
Colombo N, Dubot C, Lorusso D, et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385(20):1856–67.
Monk BJ, Colombo N, Tewari KS, et al. First-line pembrolizumab + chemotherapy versus placebo + chemotherapy for persistent, recurrent, or metastatic cervical cancer: final overall survival results of KEYNOTE-826. J Clin Oncol. 2023;41(36):5505–11.
National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in Oncology (NCCN guidelines®)—cervical cancer 2022. 2023.
Monk B, Boer J, Van Hees F, et al. Cost-effectiveness of pembrolizumab for first-line treatment in patients with persistent, recurrent, or metastatic cervical cancer in the United States. Int J Gynecol Cancer. 2022;32:A38–9.
Institute for Clinical and Economic Review. 2020–2023 value assessment framework. https://icer.org/our-approach/methods-process/value-assessment-framework/. Accessed June 10, 2024.
Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med Decis Making. 2012;32(5):722–32.
Gao W, Muston D, Monberg M, et al. A critical appraisal and recommendations for cost-effectiveness studies of poly(ADP-ribose) polymerase inhibitors in advanced ovarian cancer. Pharmacoeconomics. 2020;38(11):1201–18.
Husereau D, Drummond M, Augustovski F, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMJ. 2022;376: e067975.
Woods BS, Sideris E, Palmer S, Latimer N, Soares M. Partitioned survival and state transition models for healthcare decision making in oncology: Where are we now? Value Health. 2020;23(12):1613–21.
Muston D. Informing structural assumptions for three state oncology cost-effectiveness models through model efficiency and fit. Appl Health Econ Health Policy. 2024. https://doi.org/10.1007/s40258-024-00884-2. Online ahead of print.
Food and Drug Administration (FDA). Highlights of prescribing information: KEYTRUDA. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/125514s125lbl.pdf. Accessed June 10, 2024.
Royston P, Parmar MK. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21(15):2175–97.
Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390(10103):1654–63.
Pickard AS, Law EH, Jiang R, et al. United States valuation of EQ-5D-5L health states using an international protocol. Value Health. 2019;22(8):931–41.
Hatswell AJ, Bullement A, Schlichting M, Bharmal M. What is the impact of the analysis method used for health state utility values on QALYs in oncology? A simulation study comparing progression-based and time-to-death approaches. Appl Health Econ Health Policy. 2021;19(3):389–401.
Szende A, Janssen B, Cabases J. Self-reported population health: an international perspective based on EQ-5D [Internet]. Dordrecht: Springer. Copyright 2014; 2014.
AnalySource. AnalySource—premier drug pricing services. https://www.analysource.com/. Accessed June 10, 2024.
CMS. Physician fee schedule. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched. Accessed June 10, 2024.
Chastek B, Harley C, Kallich J, Newcomer L, Paoli CJ, Teitelbaum AH. Health care costs for patients with cancer at the end of life. J Oncol Pract. 2012;8(6):75s–80s.
Monk BJ, Enomoto T, Kast WM, et al. Integration of immunotherapy into treatment of cervical cancer: recent data and ongoing trials. Cancer Treat Rev. 2022;106: 102385.
Pike J, Grosse SD. Friction cost estimates of productivity costs in cost-of-illness studies in comparison with human capital estimates: a review. Appl Health Econ Health Policy. 2018;16(6):765–78.
Lorusso D, Colombo N, Monk BJ, et al. Pembrolizumab + chemotherapy for first-line treatment of patients with persistent, recurrent, or metastatic cervical cancer: bevacizumab subgroup analysis based on protocol-specified final overall survival results of KEYNOTE-826. Int J Gynecol Cancer. 2023;33(Suppl 3):A1.
Barrington DA, Riedinger C, Haight PJ, Tubbs C, Cohn DE. Pembrolizumab with or without bevacizumab for recurrent or metastatic cervical cancer: a cost-effectiveness analysis. Gynecol Oncol. 2022;165(3):500–5.
Shi Y, Chen J, Shi B, Liu A. Cost-effectiveness analysis of pembrolizumab for treatment of US patients with persistent, recurrent, or metastatic cervical cancer. Gynecol Oncol. 2022;164(2):379–85.
He D, Ting J, Zhang J, Monk B, Alholm Z, Sudharshan L. Real-world treatment pattern and drop-off among recurrent or metastatic cervical cancer patients: a US community oncology-based analysis (304). Presented at: Annual Meeting on Women's Cancer August 2022; Phoenix, Arizona S159.
Coleman RL, Lorusso D, Gennigens C, et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22(5):609–19.
Chung HC, Ros W, Delord JP, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2019;37(17):1470–8.
Acknowledgements
The authors would like to thank the patients and their families, as well as the investigators, study coordinators, study teams, and nurses who participated in the trials, contributing data to these analyses.
Medical Writing, Editorial, and Other Assistance
The authors would like to thank Ross Maconachie and Olga Ovcinnikova of MSD (UK) Limited, London, UK, for extensive review and critique of early versions of the economic model, and Ruifeng Xu of Merck & Co., Inc., Rahway, NJ, USA, and Abdel Hmissi of MSD, Zurich, Switzerland, for statistical analysis of the KEYNOTE-826 trial for economic modeling purposes. Editorial assistance was provided by Susan Neville, MSc, and Saba Choudhary, PhD, of Parexel, and was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Funding
Funding for this study and the cost for the journal’s Rapid Service Fee was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Author information
Authors and Affiliations
Contributions
Study conception and design were performed by Frank van Hees, Jennifer Boer, Shilpi Swami, Dominic Muston, Oliver Hale. Material preparation, data collection and analysis were performed by Sophie van Mens, Oliver Hale, Jennifer Boer, Frank van Hees, Shilpi Swami, Dominic Muston. The first draft of the manuscript was written by Bradley J. Monk, Oliver Hale, Steve Keefe, and Matthew Monberg, and all authors commented on previous versions of the manuscript. All authors, including Cumhur Tekin, commented on previous versions of the manuscript, and read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Bradley J. Monk reports consulting fees from Acrivon, Adaptimune, Agenus, Akeso Bio, Amgen, Aravive, AstraZeneca, Bayer, Clovis, Eisai, Elevar, EMD Merck, Genmab/Seagen, GOG Foundation, Gradalis, Heng Rui, ImmunoGen, Karyopharm, Iovance, Laekna, Macrogenics, Merck, Mersana, Myriad, Novartis, Novocure, OncoC4, Panavance, Pieris, Pfizer, Puma, Regeneron, Roche/Genentech, Sorrento, TESARO/GSK, US Oncology Research, VBL, Verastem, and Zentalis; and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from AstraZeneca, Eisai, Myriad, Roche/Genentech, and TESARO/GSK. Sophie van Mens, Oliver Hale, Jennifer Boer, and Frank van Hees were employees of Lumanity at the time the analysis was performed. Lumanity conducted the cost-effectiveness analysis and carried out the writing of the manuscript on behalf of MSD. Lumanity was paid by MSD as an external consultant to conduct this work. Sophie van Mens is now an employee at PharmAccess. Oliver Hale is now an employee at Delta Hat Limited. Jennifer Boer is now an employee at Equalis Strategy & Modeling BV. Frank van Hees is now an employee at Maple Health Group, LLC. Shilpi Swami was an employee of MSD (UK) Ltd., London, UK at the time the analysis was performed, who may own stock and/or stock options in Merck & Co., Inc., Rahway, NJ, USA. Dominic Muston, Cumhur Tekin, Steve Keefe, and Matthew Monberg are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, who may own stock and/or stock options in Merck & Co., Inc., Rahway, NJ, USA.
Ethical Approval
This study was based on previously collected, anonymized clinical trial data from KEYNOTE-826 provided by the study sponsor; as such, it was exempt from institutional review board or ethical approval. The KEYNOTE-826 trial was approved by the appropriate ethics body at each participating center [6, 7].
Additional information
Prior Presentation: This paper complements the interim analysis for this study which was previously presented at the International Gynecologic Cancer Society Annual Meeting (September 29–October 1, 2022, New York City, USA; Monk B, et al. Int J Gynecol Cancer. 2022;32:A38–A39).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Monk, B.J., van Mens, S., Hale, O. et al. Cost-Effectiveness of Pembrolizumab as First-Line Treatment in Patients with Persistent, Recurrent, or Metastatic Cervical Cancer in the United States. Oncol Ther 13, 85–98 (2025). https://doi.org/10.1007/s40487-024-00311-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40487-024-00311-5