Abstract
Follicular lymphoma (FL) is often considered a chronic disease with frequent relapses, shortening both response duration and survival after every relapse. Selecting the most appropriate therapy at the right time within the treatment timeline is key to optimize outcomes. The aim of this vodcast, featuring Dr. Kai Hübel, is to outline the severity of FL by referring to a patient case as well as highlight chimeric antigen receptor (CAR)-T cells as an effective therapy in relapsed/refractory (r/r) FL. The patient was in their early 50s, diagnosed with FL in the early 2010s and presented with a third relapse. The patient complained of night sweats and fatigue but was still capable of self-care (Eastern Cooperative Oncology Group Performance Status Scale 2). The patient received eight cycles of rituximab-cyclophosphamide-doxorubicin-vincristine-prednisolone (R-CHOP), followed by irradiation and rituximab maintenance (first-line) and then received rituximab 4 × weekly, followed by rituximab maintenance (second-line). The patient relapsed during rituximab maintenance; the patient was rituximab refractory. The patient received six cycles of bendamustine/obinutuzumab followed by obinutuzumab maintenance. The patient relapsed during obinutuzumab maintenance, achieved a partial remission after irradiation and was switched to R/lenalidomide. Due to several high-risk features, CAR-T cell therapy was initiated. Dr. Hubel underlines how earlier treatment with CAR-T cell therapy would have been beneficial for this patient. Results of the ELARA trial as well as comparative studies have shown tisagenlecleucel to be more effective than standard of care in extensively pretreated r/r FL, including high-risk patients. In conclusion, CAR-T cell therapy is a promising therapy option for patients with multiply r/r FL. A vodcast feature is available for this article.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Optimizing real-world outcomes in high-risk relapsed/refractory (r/r) FL with CAR-T cell therapy: a vodcast and case example (MP4 90820 KB)
Follicular lymphoma (FL) is a chronic disease with frequent relapses and certain subgroups associated with poor outcomes. |
Patients associated with poor outcomes need to be identified and allocated the appropriate treatment, with chimeric antigen receptor (CAR)-T cell therapy presenting promising data. |
CAR-T cell therapy is an effective treatment option for high-risk patients such as the patient case presented. |
Digital Features
This article is published with digital features, including a vodcast feature to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.24297499.
Introduction
My name is Kai Hübel. I'm a hematologist at the University of Cologne in Germany. The focus of my scientific interests is indolent lymphomas. I'd like to present now a case of a patient with high-risk relapse follicular lymphoma (FL).
Let me start with some background information. FL is the most frequent indolent lymphoma [1]. In the majority of patients, FL is a chronic disease with a promising overall survival (OS) [2, 3]. However, it is important to realize that FL is a relapsing disease [1]. That means response duration and survival shorten after each relapse [1]. This also means that patients may experience several treatment lines.
It was possible to identify subgroups of patients who are associated with a poor outcome, for example, patients with an early relapse [4, 5]. We call this progression of disease within 24 months (POD24) patients. That means a relapse in the first 2 years after the start of first-line therapy. Other high-risk features are a high metabolic tumor volume, double refractory patients, that means patients who are refractory to CD20 antibody and an alkylans, patients with a bulky disease, or patients with a Follicular Lymphoma International Prognostic Index (FLIPI) of 2 or higher. This group of patients may need another or more intensive treatment approach.
Case Report on FL
Let me now start with a case report. So, this is a story of a patient in their early 50s. The patient was diagnosed with FL in the early 2010s and presents with third relapse. The patient complained of night sweats, fatigue, but was still capable of all self-care. However, it was not possible to go to work. The Eastern Cooperative Oncology Group Performance status (ECOG) was 2.
In first-line, the patient received eight cycles of rituximab-cyclophosphamide-doxorubicin-vincristine-prednisolone (R-CHOP), followed by irradiation and R maintenance. This is what I think is the standard approach. Immune chemotherapy is a standard regimen for first-line treatment [6]. You may choose R-CHOP or R/bendamustine. We selected eight cycles of R-CHOP. This was part of a clinical trial. The standard is six cycles of R-CHOP. Also, the majority of patients received 2 years of R maintenance.
As second-line, the patient received R monotherapy four times, followed by R maintenance. This is, of course, not standard of care. This regimen was a patient's preference. Standard would be another immunochemotherapy or the combination of R and lenalidomide. We call this R square (R2). Our patient unfortunately relapsed during R maintenance. This means the patient is R-refractory.
So, as third-line, we selected the combination of bendamustine and obinutuzumab. Obinutuzumab is another CD20 antibody. It's a little bit more potent compared to R. After six cycles of this combination, the patient received an obinutuzumab maintenance. This was based on the data of the GADOLIN trial [7]. However, again, the patient relapsed during maintenance and achieved just a partial remission after irradiation. So, we switched to R2.
As I said before, R2 is a combination of R and lenalidomide. It is nowadays what I think is a standard treatment in relapsed/refractory (r/r) FL based on the AUGMENT trial [8]. You may use it in all treatment lines, except first line. It's my experience that it is really effective and well tolerated. However, our patient did not respond following three lines of R2.
So, in summary, our patient did not respond to fourth-line therapy. The patient has no reliable chemotherapy options. It is not possible to repeat the CHOP regimen. It makes no sense to repeat bendamustine. The patient is refractory to CD20 antibody, like obinutuzumab and R. The patient has a high FLIPI, 4 points for stage, nodal region involved, lactate dehydrogenase (LDH), and hemoglobin. So, this is what I think is a clear critical situation. There was a discussion in our hospital on how to proceed. Should we use a bispecific antibody, or should we switch the patient to chimeric antigen receptor (CAR)-T therapy? Well, both treatment approaches are really effective, but because of several high-risk features, we decided to proceed with tisa-cel. That means with the CAR-T therapy.
ELARA Trial vs. Standard of Care
Tisa-cel was approved based on the ELARA trial [4]. The ELARA trial is a phase II trial focusing on patients with relapsed follicular lymphoma [4]. The trial includes patients with high-risk features. The progression-free survival (PFS) for the overall population in the trial at 12 months was 67% [4]. However, it was much higher for patients who achieved a complete remission. For this group of patients, the PFS at 12 months was 85.5% [4]. The median duration of response, PFS, OS, and time till next anti-lymphoma treatment were not reached.
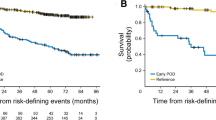
There is some concern regarding the development of a cytokine release syndrome (CRS) or neurological events. In the ELARA trial, nearly half of the patients developed a CRS [4]. However, no patient developed a severe CRS. That means a CRS grade 3 or higher. Just three patients developed a grade 3 or higher neurological event. High-risk subgroups are patients with POD24, high metabolic tumor volume, bulky disease, double refractory patients, or patients with a high FLIPI [4]. What you can see here in all of these situations is a really high overall response rate [4], for high metabolic tumor volume, 75%; in all other situations, more than 80%. There is also a really high number of patients who achieve a complete remission.
Unfortunately, we have no head-to-head comparison between tisa-cel and other treatment approaches. So, it could make sense to compare the data of the ELARA trial with data from registry; for example, with the ReCORD-FL chart review. In this registry, patients were treated out of a clinical trial. That means real-world data were collected. Of course, you cannot easily compare both data. There was a matching performed based on baseline characteristics. Finally, 99 patients from the ReCORD-FL registry were compared to 97 patients in the ELARA trial [4, 9]. Again, these patients have the same baseline characteristics.
The overall response rate was 85.6% in the ELARA trial compared to just 63% in a real-world setting. There was also a significantly higher complete response (CR) rate in the ELARA trial compared to the ReCORD registry [4, 9]. So again, tisa-cel was associated with an improvement over standard of care in CR and overall response rate after weighted adjustment for baseline variables.
What is the survival? The median PFS and event-free survival (EFS) were not reached for tisa-cel recipients in the ELARA trial [4]. However, it was just 13.1 months for usual care patients and ReCORD-FL registry [4, 9]. The OS at 12 months was 96.6% for tisa-cel compared to 71.7% for the patients in the registry [4, 9]. That means tisa-cel demonstrated superior efficiency to the standard-of-care patients with r/r FL. Please keep in mind this was an indirect comparison. This was a retrospective comparison with retrospective data. In the registry, there were no modern treatments recorded, for example, patients who received bispecific antibodies. So, these are clear limitations.
The median time to next treatment, OS, and PFS, or event-free survival (EFS) was not reached in all situations for the patients in the ELARA trial. For 24 months time to next treatment, OS, PFS, and EFS [4, 9] showed a clear advantage favoring the use of tisa-cel compared to standard of care.
The Patient Case (Continued)
So back to the patient. The patient has undergone tisa-cel therapy. Overall, the patient responded well. The patient had no severe side effects, is still in remission 6 months after CARs, and—what is, I think, very important—had a possibility to return to work.
Before CAR-T cells, the patient had enlarged lymph nodes. Three months after CARs, the Deauville score was just 2. That means the patient became PET-negative.
So, in summary, this case report highlights the effectiveness of CARs in r/r FL. This is just a case report. That means result may be difficult to replicate, but I think it's a very, very nice example of how effective CAR-T cells are in high-risk FL. So, the question is, would earlier treatment with CARs have been beneficial for this patient? I think, yes. Tisa-cel is approved after two relapses. So, what I recommend is to use, in such a patient, tisa-cel after two relapses instead of bendamustine and obinutuzumab. The final question is, what is the future of CAR-T cells? Would it make sense to use them in earlier treatment lines, for example, in first relapse? I think, yes. For example, patients with POD24, as I said at the beginning, have a poor outcome. It really makes sense to analyze whether CAR-T cells are effective in this situation. The trials have already started.
Thank you very much for your attention.
References
Rivas-Delgado A, Magnano L, Moreno-Velázquez M, et al. Response duration and survival shorten after each relapse in patients with follicular lymphoma treated in the rituximab era. Br J Haematol. 2019;184:753–9.
Casulo C, Byrtek M, Dawson KL, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: An analysis from the National LymphoCare Study. J Clin Oncol. 2015;33:2516–22.
Tan D, Horning SJ, Hoppe RT, Levy R, Rosenberg SA, Sigal BM. Improvements in observed and relative survival in follicular grade 1–2 lymphoma during 4 decades: the Stanford University experience. Blood. 2013;122:981–7.
Fowler NH, Dickinson M, Dreyling M, et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med. 2022;28:325–32.
Casulo C. Upfront identification of high-risk follicular lymphoma. Hemat Oncol. 2021;39:88–93.
Dreyling M, Ghielmini M, Rule S, et al. Newly diagnosed and relapsed follicular lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32:298–308.
Sehn LH, Chua N, Mayer J, Dueck G, Trněný M, Bouabdallah K. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (Gadolin): a randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol. 2016;17:1081–93.
Leonard JP, Trneny M, Izutsu K, et al. Augment: a phase III study of lenalidomide plus rituximab versus placebo plus rituximab in relapsed or refractory indolent lymphoma. J Clin Oncol. 2019;37:1188–99.
Salles G, Schuster SJ, Dreyling M, Fischer L, Kuruvilla J, Patten EMP. Efficacy comparison of tisagenlecleucel vs usual care in patients with relapsed or refractory follicular lymphoma. Blood Adv. 2022;6:5835–43.
Acknowledgements
Author Contribution.
Dr. Kai Hübel meets the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, takes responsibility for the integrity and accuracy of the work, and has given his review and approval for the final vodcast recording, discussion guide, slides, and transcript.
Funding.
Novartis Pharma AG provided funding for the creation and publication of the vodcast commentary. The journal’s Rapid Service fee was also funded by Novartis Pharma AG.
Editorial Assistance.
Editorial assistance with the preparation of the vodcast discussion guide and abstract was provided by Chloe Schon and Jonathon Ackroyd, Springer Healthcare Ltd., UK. Funding for this assistance was provided by Novartis Pharma AG.
Data Availability.
Data sharing is not applicable to this vodcast as no datasets were generated or analyzed during the vodcast.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Kai Hübel: Consultant: AbbVie, Recordati, Gilead, Incyte, Novartis, Roche; Speaker’s Bureau: AbbVie, BeiGene, Recordati, Incyte, Sanofi, Roche; Scientific Advisory Board: AbbVie, Roche, Sanofi, BMS, Recordati, Novartis, Miltenyi Biotec; Research Support: Roche, Gilead, Incyte.
Ethical Approval
Ethical approval was not applicable for this vodcast.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hübel, K. Optimizing Real-World Outcomes in High-Risk Relapsed/Refractory (r/r) FL with CAR-T Cell Therapy: A Vodcast and Case Example. Oncol Ther (2024). https://doi.org/10.1007/s40487-024-00269-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40487-024-00269-4