Abstract
Purpose
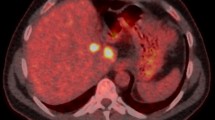
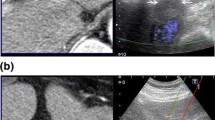
To report our first results on sixteen patients affected by liver and abdominal malignant tumors, unfit for surgery or thermal ablation, treated with US-guided percutaneous irreversible electroporation (IRE).
Methods
From June 2014 to December 2016, all patients meeting the inclusion criteria (malignant hepatic or abdominal tumors not eligible for resection or thermal ablation) and not meeting the exclusion criteria (heart arrhythmia, pro-hemorrhagic hematological alterations, tumor size > 8 cm, presence of a biliary metallic stent) referred to our institutions were prospectively enrolled to undergo percutaneous US-guided irreversible electroporation (IRE). Sixteen patients (age range 59–68 years, mean 63; 7 females) with 18 tumors (diameter range 1.3–7.5 cm) fulfilled the inclusion criteria and were included in the study. Data concerning efficacy (tested by a 1-week CEUS and a 4-week enhanced CT and/or enhanced MRI) and safety were recorded during a 18-month follow up.
Results
All patients completed a 35–50-min procedure without complications. One patient with 6 cm Klatskin tumor also underwent a second session for 1 month. A 1-week CEUS and a 4-week e-CT and/or e-MRI arterial phase contrast enhancement analysis showed an overall reduction of arterial flow with confirmation of unenhanced lesions for seven nodules. After 1–18 months of follow up, no major complications were recorded and no tumor-related death occurred. The lesions of two patients disappeared 3 and 6 months after their treatment, respectively.
Conclusions
IRE is a promising ablation modality in the treatment of malignant hepatic and abdominal tumors unsuitable for resection or thermal ablation.


Similar content being viewed by others
References
Narayanan G, Froud T, Suthar R, Barbery K (2013) Irreversible electroporation of hepatic malignancy. Semin Interv Radiol 30:67–73. https://doi.org/10.1055/s-0033-1333655
Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH (1982) Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J 1:841–845
Okino M, Mohri H (1987) Effects of a high-voltage electrical impulse and an anticancer drug on in vivo growing tumors. Jpn J Cancer Res 78:1319–1321
Davalos RV, Mir IL, Rubinsky B (2005) Tissue ablation with irreversible electroporation. Ann Biomed Eng 33:223–231
Lee EW, Chen C, Prieto VE, Dry SM, Loh CT, Kee ST (2010) Advanced hepatic ablation technique for creating complete cell death: irreversible electroporation. Radiology 255:426–433
Thomson KR, Cheung W, Ellis SJ et al (2011) Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol 22:611–621
Rubinsky B, Onik G, Mikus P (2007) Irreversible electroporation: a new ablation modality—clinical implications. Technol Cancer Res Treat 6:37–48
Dollinger M et al (2015) Adverse effects of irreversible electroporation of malignant liver tumors under CT fluoroscopic guidance: a single-center experience. Diagn Interv Radiol 21(6):471–475. https://doi.org/10.5152/dir.2015.14442
Niessen C et al (2016) Percutaneous ablation of hepatic tumors using irreversible electroporation: a prospective safety and midterm efficacy study in 34 patients. J Vasc Interv Radiol 27(4):480–486. https://doi.org/10.1016/j.jvir.2015.12.025 (Epub 2016 Feb 26)
Distelmaier M et al (2017) Midterm safety and efficacy of irreversible electroporation of malignant liver tumors located close to major portal or hepatic veins. Radiology 285(3):1023–1031. https://doi.org/10.1148/radiol.2017161561 (Epub 2017 Aug 11)
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30:52–60. https://doi.org/10.1055/s-0030-1247132
Lyu T, Wang X, Su Z, Shangguan J, Sun C et al (2017) Irreversible electroporation in primary and metastatic hepatic malignancies: a review. Medicine (Baltimore) 96:e6386. https://doi.org/10.1097/MD.0000000000006386
Sugimoto K, Moriyasu F, Kobayashi Y, Saito K, Takeuchi H et al (2015) Irreversible electroporation for nonthermal tumor ablation in patients with hepatocellular carcinoma: initial clinical experience in Japan. Jpn J Radiol 33:424–432. https://doi.org/10.1007/s11604-015-0442-1
Cheng RG, Bhattacharya R, Yeh MM, Padia SA (2015) Irreversible electroporation can effectively ablate hepatocellular carcinoma to complete pathologic necrosis. J Vasc Interv Radiol 26:1184–1188. https://doi.org/10.1016/j.jvir.2015.05.014
Ball C, Thomson KR, Kavnoudias H (2010) Irreversible electroporation: a new challenge in “out of operating theater” anesthesia. Anesth Analg 110:1305–1309. https://doi.org/10.1213/ANE.0b013e3181d27b30
Niessen C, Igl J, Pregler B, Beyer L, Noeva E et al (2015) Factors associated with short-term local recurrence of liver cancer after percutaneous ablation using irreversible electroporation: a prospective single-center study. J Vasc Interv Radiol 26:694–702. https://doi.org/10.1016/j.jvir.2015.02.001
Giorgio A, Tarantino L, de Stefano G, Perrotta A, Aloisio V, del Viscovo L et al (2000) Ultrasound-guided percutaneous ethanol injection under general anesthesia for the treatment of hepatocellular carcinoma on cirrhosis: long-term results in 268 patients. Eur J Ultrasound 12:145–154
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Informed consent
The institutional review board approved the study and all patients gave their informed written consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Giorgio, A., Amendola, F., Calvanese, A. et al. Ultrasound-guided percutaneous irreversible electroporation of hepatic and abdominal tumors not eligible for surgery or thermal ablation: a western report on safety and efficacy. J Ultrasound 22, 53–58 (2019). https://doi.org/10.1007/s40477-019-00372-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40477-019-00372-7