Abstract
Purpose of Review
There are several aspects of donor selection for heart transplantation that are particularly challenging. Information about the donor is often limited and relayed through a review of selected chart snippets and tests. The donor heart must work immediately unlike kidney transplantation where delayed function is common and not life-threatening. This review focuses on recent findings in the literature which are distilled for the transplant clinician to be eminently practical to advise teams as they consider which donor factors are important in individual situations.
Recent Findings
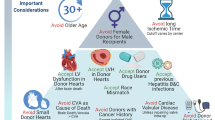
Left ventricular dysfunction has many causes and particularly in younger donors may resolve with time. Such donors can be utilized with excellent results. Donor age is still a critically important risk factor for outcome post-transplant though it is additive with other factors such as left ventricular hypertrophy and anticipated ischemic time. Donor size is another important factor and recent work suggests that calculation of predicted heart mass is superior to simple weight, height, and gender in reaching a decision about donor sizing. Recent work suggests that even donors with non-critical coronary lesions (50% or less) may be utilized with similar outcomes, including a similar incidence of progression of vasculopathy. Diabetes appears to be a manageable comorbidity particularly with careful screening. With the increase in drug-related deaths, a recent study looking at more than 23,000 accepted donors found no difference in long-term survival with multiple drugs in the toxicology screen of the donor which may allow increased use of such donors. Finally, we review the details of donation after circulatory determination of death (DCD). Currently, this is confined to certain specialized centers but this is anticipated to become more common worldwide and enhance the number of transplants over time.
Summary
Current evidence is reviewed and summarized to allow the busy transplant clinician to be up to date on the latest information regarding large and smaller important studies in this field. It is hoped that this review helps teams maximize their use of appropriate donors.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Dujardin KS, McCully RB, Wijdicks EF, et al. Myocardial dysfunction associated with brain death: clinical, echocardiographic, and pathologic features. J Heart Lung Transplant. 2001;20:350–7.
Zaroff JG, Babcock WD, Shiboski SC. The impact of left ventricular dysfunction on cardiac donor transplant rates. J Heart Lung Transplant. 2003;22:334–7.
Bombardini T, Arpesella G, Maccherini M, et al. Medium-term outcome of recipients of marginal donor hearts selected with new stress-echocardiographic techniques over standard criteria. Cardiovasc Ultrasound. 2014;12:20.
Garcia-Dorado D, Andres-Villarreal M, Ruiz-Meana M, Inserte J, Barba I. Myocardial edema: a translational view. J Mol Cell Cardiol. 2012;52:931–9.
Oras J, Doueh R, Norberg E, Redfors B, Omerovic E, Dellgren G. Left ventricular dysfunction in potential heart donors and its influence on recipient outcomes. J Thorac Cardiovasc Surg. 2020;159:1333-1341.e6.
Poptsov V, Khatutskiy V, Skokova A, et al. Heart transplantation from donors with left ventricular ejection fraction under forty percent. Clin Transplant. 2021;35:e14341. The authors report use of highly selected heart donors with LVEF under 40 %. Of 47 hearts examined, 27 were used for transplant with good results. This illustrates that some dysfunctional donors can be utilized by experienced teams with close scrutiny of the donor and meticulous post-operative support.
Madan S, Saeed O, Vlismas P, et al. Outcomes after transplantation of donor hearts with improving left ventricular systolic dysfunction. J Am Coll Cardiol. 2017;70:1248–58.
Bombardini T, Gherardi S, Leone O, Sicari R, Picano E. Transplant of stunned donor hearts rescued by pharmacological stress echocardiography: a “proof of concept” report. Cardiovasc Ultrasound. 2013;11:27.
Kono T, Nishina T, Morita H, Hirota Y, Kawamura K, Fujiwara A. Usefulness of low-dose dobutamine stress echocardiography for evaluating reversibility of brain death-induced myocardial dysfunction. Am J Cardiol. 1999;84:578–82.
Axtell AL, Fiedler AG, Chang DC, et al. The effect of donor age on posttransplant mortality in a cohort of adult cardiac transplant recipients aged 18–45. Am J Transplant. 2019;19:876–83.
Smits JM, De Pauw M, de Vries E, et al. Donor scoring system for heart transplantation and the impact on patient survival. J Heart Lung Transplant. 2012;31:387–97.
Sabatino M, Vitale G, Manfredini V, et al. Clinical relevance of the International Society for Heart and Lung Transplantation consensus classification of primary graft dysfunction after heart transplantation: Epidemiology, risk factors, and outcomes. J Heart Lung Transplant. 2017;36:1217–25.
Lund LH, Khush KK, Cherikh WS, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Heart Transplantation Report-2017; Focus Theme: Allograft ischemic time. J Heart Lung Transplant. 2017;36:1037–46.
Goff RR, Uccellini K, Lindblad K, et al. A change of heart: preliminary results of the US 2018 adult heart allocation revision. Am J Transplant. 2020;20:2781–90.
Hsich EM, Blackstone EH, Thuita LW, et al. Heart transplantation: an in-depth survival analysis. JACC Heart Fail. 2020;8:557–68.
Jawitz OK, Fudim M, Raman V, et al. Reassessing recipient mortality under the new heart allocation system: an updated UNOS registry analysis. JACC Heart Fail. 2020;8:548–56.
Hoffman JRH, Larson EE, Rahaman Z, et al. Impact of increased donor distances following adult heart allocation system changes: a single center review of 1-year outcomes. J Card Surg. 2021;36:3619–28.
Kilic A, Mathier MA, Hickey GW, et al. Evolving trends in adult heart transplant with the 2018 heart allocation policy change. JAMA Cardiol. 2021;6:159–67.
Lebreton G, Coutance G, Bouglé A, Varnous S, Combes A, Leprince P. Changes in heart transplant allocation policy: “unintended” consequences but maybe not so “unexpected….” Asaio j. 2021;67:e69–70.
Patel JN, Chung JS, Seliem A, et al. Impact of heart transplant allocation change on competing waitlist outcomes among listing strategies. Clin Transplant. 2021;35:e14345.
Baran DA. How to save a life: ex vivo heart preservation. Asaio j. 2021;67:869–70.
Costanzo MR, Dipchand A, Starling R, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29:914–56.
Martinez-Selles M, Almenar L, Paniagua-Martin MJ, et al. Donor/recipient sex mismatch and survival after heart transplantation: only an issue in male recipients? An analysis of the Spanish Heart Transplantation Registry. Transpl Int. 2015;28:305–13.
Khush KK, Kubo JT, Desai M. Influence of donor and recipient sex mismatch on heart transplant outcomes: analysis of the International Society for Heart and Lung Transplantation Registry. J Heart Lung Transplant. 2012;31:459–66.
Kaczmarek I, Meiser B, Beiras-Fernandez A, et al. Gender does matter: gender-specific outcome analysis of 67,855 heart transplants. Thorac Cardiovasc Surg. 2013;61:29–36.
Reed RM, Netzer G, Hunsicker L, et al. Cardiac size and sex-matching in heart transplantation : size matters in matters of sex and the heart. JACC Heart Fail. 2014;2:73–83.
Bergenfeldt H, Stehlik J, Höglund P, Andersson B, Nilsson J. Donor-recipient size matching and mortality in heart transplantation: influence of body mass index and gender. J Heart Lung Transplant. 2017;36:940–7.
Kransdorf EP, Kittleson MM, Benck LR, et al. Predicted heart mass is the optimal metric for size match in heart transplantation. J Heart Lung Transplant. 2019;38:156–65. This paper established the utility of predicted heart mass generated by calculations and proved its relation to post transplant outcomes.
Wever Pinzon O, Stoddard G, Drakos SG, et al. Impact of donor left ventricular hypertrophy on survival after heart transplant. Am J Transplant. 2011;11:2755–61.
Goland S, Czer LS, Kass RM, et al. Use of cardiac allografts with mild and moderate left ventricular hypertrophy can be safely used in heart transplantation to expand the donor pool. J Am Coll Cardiol. 2008;51:1214–20.
Marelli D, Laks H, Fazio D, Moore S, Moriguchi J, Kobashigawa J. The use of donor hearts with left ventricular hypertrophy. J Heart Lung Transplant. 2000;19:496–503.
Lechiancole A, Vendramin I, Sponga S, et al. Influence of donor-transmitted coronary artery disease on long-term outcomes after heart transplantation - a retrospective study. Transpl Int. 2021;34:281–9. The authors demonstrate excellent long-term outcomes for hearts with coronary artery disease as compared to donors without coronary disease from a single European center. They illustrate that fears of CAD progressing rapidly in donors with existing disease is not confirmed in practice.
Taghavi S, Jayarajan SN, Wilson LM, Komaroff E, Testani JM, Mangi AA. Cardiac transplantation can be safely performed using selected diabetic donors. J Thorac Cardiovasc Surg. 2013;146:442–7.
Fluschnik N, Geelhoed B, Becher PM, et al. Non-immune risk predictors of cardiac allograft vasculopathy: results from the U.S. organ procurement and transplantation network. Int J Cardiol. 2021;331:57–62.
Desai R, Collett D, Watson CJ, Johnson P, Evans T, Neuberger J. Cancer transmission from organ donors-unavoidable but low risk. Transplantation. 2012;94:1200–7.
Doerfler A, Tillou X, Le Gal S, Desmonts A, Orczyk C, Bensadoun H. Prostate cancer in deceased organ donors: a review. Transplant Rev (Orlando). 2014;28:1–5.
Kaul DR, Vece G, Blumberg E, et al. Ten years of donor-derived disease: a report of the disease transmission advisory committee. Am J Transplant. 2021;21:689–702.
Jayarajan S, Taghavi S, Komaroff E, et al. Long-term outcomes in heart transplantation using donors with a history of past and present cocaine use. Eur J Cardiothorac Surg. 2015;47:e146–50.
Vieira JL, Cherikh WS, Lindblad K, Stehlik J, Mehra MR. Cocaine use in organ donors and long-term outcome after heart transplantation: an International Society for Heart and Lung Transplantation registry analysis. J Heart Lung Transplant. 2020;39:1341–50.
Civelli VF, Sharma R, Sharma O, Sharma P, Heidari A, Singh S. Methamphetamine-induced Takotsubo cardiomyopathy with hypotension, resolved by low-dose inotropes. Am J Ther. 2019;28:e498–501.
Schwarzbach V, Lenk K, Laufs U. Methamphetamine-related cardiovascular diseases. ESC. Heart Fail. 2020;7:407–14.
Varian KD, Gorodeski EZ. The other substance abuse epidemic: methamphetamines and heart failure. J Card Fail. 2020;26:210–1.
Zhao SX, Seng S, Deluna A, Yu EC, Crawford MH. Comparison of clinical characteristics and outcomes of patients with reversible versus persistent methamphetamine-associated cardiomyopathy. Am J Cardiol. 2020;125:127–34.
Baran DA, Lansinger J, Long A et al. Intoxicated donors and heart transplant outcomes: long-term safety. Circ Heart Fail 2021:Circheartfailure120007433. This is the only paper to look at the toxicology of donors from the UNOS data set and conclusively shows that donor drug use in otherwise acceptable donors is associated with similar long-term survival.
Taghavi S, Jayarajan SN, Komaroff E, et al. Use of heavy drinking donors in heart transplantation is not associated with worse mortality. Transplantation. 2015;99:1226–30.
Kobashigawa J, Khush K, Colvin M, et al. Report From the American Society of Transplantation Conference on Donor Heart Selection in Adult Cardiac Transplantation in the United States. Am J Transplant. 2017;17:2559–66.
Hussain Z, Yu M, Wozniak A, et al. Impact of donor smoking history on post heart transplant outcomes: a propensity-matched analysis of ISHLT registry. Clin Transplant. 2021;35:e14127.
Loupy A, Coutance G, Bonnet G, et al. Identification and characterization of trajectories of cardiac allograft vasculopathy after heart transplantation: a population-based study. Circulation. 2020;141:1954–67.
Aslam S, Grossi P, Schlendorf KH, et al. Utilization of hepatitis C virus-infected organ donors in cardiothoracic transplantation: an ISHLT expert consensus statement. J Heart Lung Transplant. 2020;39:418–32.
Kahn JA. The use of organs from hepatitis C virus-viremic donors into uninfected recipients. Curr Opin Organ Transplant. 2020;25:620–5.
Kilic A, Hickey G, Mathier M, et al. Outcomes of adult heart transplantation using hepatitis C-positive donors. J Am Heart Assoc. 2020;9:e014495.
Reyentovich A, Gidea CG, Smith D, et al. Outcomes of the treatment with glecaprevir/pibrentasvir following heart transplantation utilizing hepatitis C viremic donors. Clin Transplant. 2020;34:e13989.
Schlendorf KH, Zalawadiya S, Shah AS, et al. Expanding heart transplant in the era of direct-acting antiviral therapy for hepatitis C. JAMA Cardiol. 2020;5:167–74.
Smith DE, Chen S, Fargnoli A, et al. Impact of early initiation of direct-acting antiviral therapy in thoracic organ transplantation from hepatitis C virus positive donors. Semin Thorac Cardiovasc Surg. 2021;33:407–15.
Gidea CG, Narula N, Reyentovich A et al. Increased early acute cellular rejection events in hepatitis C-positive heart transplantation. J Heart Lung Transplant 2020.
Madan S, Patel SR, Jorde UP. Cardiac allograft vasculopathy and secondary outcomes of hepatitis C-positive donor hearts at 1 year after transplantation. J Heart Lung Transplant 2020.
Dhital KK, Iyer A, Connellan M, et al. Adult heart transplantation with distant procurement and ex-vivo preservation of donor hearts after circulatory death: a case series. Lancet. 2015;385:2585–91.
Iyer A, Chew HC, Gao L, et al. Pathophysiological trends during withdrawal of life support: implications for organ donation after circulatory death. Transplantation. 2016;100:2621–9.
White CW, Lillico R, Sandha J, et al. Physiologic changes in the heart following cessation of mechanical ventilation in a porcine model of donation after circulatory death: implications for cardiac transplantation. Am J Transplant. 2016;16:783–93.
Scheuer SE, Jansz PC, Macdonald PS. Heart transplantation following donation after circulatory death: expanding the donor pool. J Heart Lung Transplant 2021.
Xu G, Guo Z, Liang W, et al. Prediction of potential for organ donation after circulatory death in neurocritical patients. J Heart Lung Transplant. 2018;37:358–64.
Messer S, Cernic S, Page A, et al. A 5-year single-center early experience of heart transplantation from donation after circulatory-determined death donors. J Heart Lung Transplant. 2020;39:1463–75.
Chew HC, Iyer A, Connellan M, et al. Outcomes of donation after circulatory death heart transplantation in Australia. J Am Coll Cardiol. 2019;73:1447–59. This is a comprehensive report about the DCD heart program in Sydney, Australia with comparison to non-DCD heart transplants.
Nadel J, Scheuer S, Kathir K, Muller D, Jansz P, Macdonald P. Successful transplantation of high-risk cardiac allografts from DCD donors following ex vivo coronary angiography. J Heart Lung Transplant. 2020;39:1496–9.
Villanueva JE, Chew HC, Gao L, et al. The effect of increasing donor age on myocardial ischemic tolerance in a rodent model of donation after circulatory death. Transplant Direct. 2021;7:e699.
Copeland H, Hayanga JWA, Neyrinck A, et al. Donor heart and lung procurement: a consensus statement. J Heart Lung Transplant. 2020;39:501–17.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Copeland reports receiving consulting fees from Bridge to Life, and her spouse is a paid consultant for Syncardia. Dr. Baran reports consulting fees from Abiomed, Getinge, Livanova, and Abbott. He is on the Steering Committee for CareDx and Procyrion. He is a speaker for Pfizer. None of the authors has conflicts to report.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baran, D.A., Mohammed, A., Macdonald, P. et al. Heart Transplant Donor Selection: Recent Insights. Curr Transpl Rep 9, 12–18 (2022). https://doi.org/10.1007/s40472-022-00355-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40472-022-00355-4