Abstract
Introduction
This study will test the hypothesis that primary aldosteronism (PA) involves alterations in Na+, K+, and water content in the skin that are corrected by adrenalectomy.
Aim and Methods
In skin biopsies, we will measure the content of Na+, K+, water, by physical-chemical methods and the osmotic-stress-responsive transcription factor Tonicity-responsive Enhancer Binding Protein (TonEBP, NFAT5) mRNA copy number by droplet digital PCR, in sex-balanced cohorts of 18 -75-year-old consecutive consenting patients with unilateral and bilateral PA, primary (essential) hypertension, and normotension. Before surgery, the patients with unilateral PA will receive the mineralocorticoid receptor antagonist (MRA) canrenone at doses that correct hypokalemia and high blood pressure values. They will be reassessed in an identical way one month after surgical cure, while off MRA. PA patients not selected for adrenalectomy will similarly be assessed at diagnosis and follow-up while on stable MRA treatment. Since a pilot study showed a direct correlation of dry weight (DW) with skin electrolytes and water content and significant differences of biopsy DW between surgery and follow-up, meaningful comparison of the skin cations and water content and TonEBP mRNA copy number, between specimen obtained at different time points, will require DW- and total mRNA-adjustment, respectively.
Conclusion
This study will provide novel information on the skin Na+, K+ and water content in PA, the paradigm of salt-dependent hypertension, and novel knowledge on the effect of surgical cure of hyperaldosteronism. The TonEBP-mediated regulation of Na+, K+ and water content in the skin will also be unveiled.
Trail Registry
Trial Registration number: NCT06090617. Date of Registration: 2023-10-19.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Sodium (Na+) is the major determinant of body fluid volumes and plays a substantial role in raising blood pressure (BP) values, thereby causing arterial hypertension (HT) (for review [1, 2]). According to recent views, the majority of Na+ is contained in the interstitial fluid of tissues and organs [3], including the heart, where expansion of salt and water content can detrimentally affect their function [4]. Hence, assessing the Na+ and water content in tissues is an important field of research where available data are yet limited.
Of note, 30% of total body Na+ content is located in the interstitial space, including that of the skin, which, therefore, represents a Na+ reservoir [3]. However, even though the skin is a relatively easily accessible tissue where to measure the Na+, K+, and water content, only scant data exist thus far. In fact, the only study available has investigated heterogenous series of patients, who were on treatment with multiple antihypertensive agents [5], many of which, as diuretics, and blockers of the renin-angiotensin system, are known to affect renal handling and, therefore, body content of Na+ [6].
We have herein developed a protocol registered at Clinicaltrial.gov [NCT06090617] that could help in avoiding the common biases that affect the findings and their interpretation when investigating the skin content of Na+, K+, and water. This was originally conceived to be applied to primary aldosteronism (PA), because it represents the prototype of salt-dependent human HT, and is surgically curable in the vast majority of the patients, who have unilateral PA. Accordingly, re-investigation of the PA patients after surgical cure will allow to infer a causal relationship between hyperaldosteronism and the water and electrolyte content abnormalities that will eventually be detected. This study design pays utmost attention to the potential effect of confounders and can easily be implemented to investigate other forms of human HT and, by at large, cardiovascular conditions where Na+ is held to play an important role as, for example, heart failure.
2 Materials and Methods
2.1 Aims and Primary Endpoints
The primary goal of this study is to test the hypothesis that the Na+, K+, and water content in the skin, is altered in PA, the prototype of salt-dependent hypertension. We will also test the hypothesis that these alterations are corrected by surgical cure of PA. Additional goals are to determine if there is more Na+ and less K+ deposition in PA patients as compared to hypertensive patients without PA, and to healthy normotensive controls and if these changes are associated with altered expression of Tonicity-responsive Enhancer Binding Protein (TonEBP, NFAT5) mRNA copy number, taken as an index of tissue osmotic stress. Besides TonEBP expression, also its cellular localization (cytoplasmic or nuclear), and the resulting protein expression will be assessed. To this end, molecules involved in the VEGF-C/VEGFR3/ERK-AKT pathway will be quantified as indicators of enhanced neo-lympho-angiogenesis [5].
2.2 Study Design
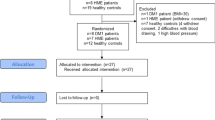
This study was conceived as a within-patient prospective observational clinical study. Its schematic layout is shown in Fig. 1 along with its primary endpoints. Four study arms will be considered: (1) PA group 1: conclusive diagnosis of unilateral PA (uPA); (2) PA group 2: presumed diagnosis of bilateral PA (bilPA) [7]; (3) PH Group 3: primary (essential) hypertension (PH); (4) Group 4: normotensive control patients undergoing surgery for benign conditions other than cardiovascular disease.
The scheme illustrates the study design and the Groups of patients who will be recruited for the within-patient and cross-sectional comparison of electrolytes, water and NFAT5 (TonEBP) content in the skin. AVS adrenal veins sampling, uPA unilateral primary aldosteronism, bPA bilateral primary aldosteronism, PHT primary (essential) hypertension, NT normotensive patients
In Group 1, to investigate the effect of uPA cure, the Na+, K+, and water skin content in the biopsy obtained during adrenalectomy will be within-patient compared to that obtained one month after adrenalectomy. In Group 2 comprising bilPA patients, the first biopsy obtained during AVS (adrenal vein sampling) will be compared to that obtained after one month of continued medical treatment with an unchanged dose of canrenone. The hypertensive patients in Group 3 and the normotensive patients in Group 4 will be used for a cross-sectional comparison of electrolytes, water and mRNA content with the PA patients in Groups 1 and 2.
All procedures will be performed according to good clinical practice and to the Declaration of Helsinki and the study will start after approval of the Ethics Committee of the University of Padova.
2.3 Inclusion Criteria
We will recruit consecutive consenting patients, aged from 18 to 75 years, with known arterial hypertension and/or treatment with antihypertensive drugs, as confirmed by daytime ambulatory BP monitoring (ABPM) or home blood pressure measurement. For ABPM the cutoff values will be < 130/80 mmHg; for home BP they will be ≥ 140 mmHg for systolic and/or ≥ 90 mmHg for diastolic.
PA will be diagnosed according to current guidelines [6] based on plasma aldosterone concentration (PAC) > 15 ng/dL and aldosterone/renin ratio greater (ARR) than 20.6 ng/mIU, while on a 100–300 mEq daily Na+ intake, as verified by 24-h urinary Na+ excretion. To achieve wash-out of interfering drugs, PAC and renin will be measured after drug treatment switch. PA will be diagnosed as unilateral (uPA) or bilateral (bilPA) based on adrenal vein sampling, as described [8] .
The uPA patients will be treated with adrenalectomy of the culprit adrenal gland and reassessed at follow-up after surgical cure and MRA withdrawal. Normalization of PAC and the ARR will be used to confirm biochemical cure. The bilPA patients will also be reassessed at follow-up while they will continue MRA treatment (Fig. 1).
Primary (essential) hypertension patients forming the PH Group will be enrolled after exclusion of PA or any other secondary form of hypertension, as described in detail [9].
Consenting healthy patients of similar age with normal arterial blood pressure values and no use of any anti-hypertensive drugs will be recruited.
2.4 Exclusion Criteria
The main exclusion criteria will be: (1) history of allergy/intolerance to local anesthesia; (2) refusal of the patient to undergo skin biopsy; (3) refusal of the patient to undergo AVS, and/or contraindications to the general anesthesia (required for laparoscopic adrenalectomy) and/or to undergo adrenalectomy, if indicated; (4) cortisol–aldosterone co-secreting adenoma or any forms of secondary HT other than PA. Additional exclusion criteria are listed in Table 1.
2.5 Skin Biopsy Procedure
In the patients with PA, the skin biopsy will be performed either during AVS or during adrenalectomy (in Group 1, uPA), and repeated one-month after surgery or continued MRA treatment (in Group 2 bilPA) patients. In the PH patients (Group 3 PH) and in the normotensive control patients (Group 4) the skin biopsy will be done only once during surgery for benign diseases (Fig. 2).
Skin biopsies will be obtained using a disposable sterile biopsy punch (KAI MEDICAL BP-40F, ø 4 mm) at the beginning of the procedure with the utmost care to prevent contamination with Na+ and K+ containing agents. To this end, we will use chlorhexidine and alcohol-based solution (such as NEOXINAL and FARVICETT, Nuova Farmec Srl) to ensure the sterilization of the surgical field. As local anesthetic for the biopsies at follow-up in the outpatient setting, a lidocaine-based cream containing no Na+ or K+ (LMX4 cream, Lidocaine 4%, Ferndale Pharmaceuticals Ltd) will be used. It will be removed with a swift sweep with Na+/K+-free 70% alcohol wipes before performing the procedure.
The collected skin samples will be cut into pieces and stored as follows: the first two samples will be immediately put in a cryo-vial and frozen in liquid nitrogen for electrolyte measurement and biomolecular analysis respectively, while the third piece will be fixed in formalin for histologic analysis (Fig. 2).
We will use physical-chemical methods to measure the Na+, K+, and water content in the skin of patients with PA, primary (essential) hypertension HT patients (PH Group) and in normotensive patients (Control Group). To these aims, all frozen samples will be handled and cut in a cold lab chamber (4 °C) in order to ensure that the samples will be of comparable size before weighting. During this process the cryo-vials (containing the skin samples) will be kept and immediately put back in thermo-insulated cryogenic store boxes containing dry ice to minimize the water loss caused by exposition to open air. They will be then weighed to determine total (WET) weight and then desiccated at 90 °C for 72 h to determine the dry weight (DW). In a set of preliminary experiments, it was previously found that after this period the sample weight remains practically unchanged. Estimated water content will be calculated as difference between total WET and DW.
The measurement of Na+ and K+ in the skin will be carried out as follows: homogenates of dry skin will be suspended overnight in 1N nitric acid to displace Na+ and K+. The supernatant will then be diluted as needed to achieve a concentration that allows analyzing through atomic adsorption spectro-photometer (SpectrAA 50/55 Varian, Agilent), in the proper working range of the machine. Reading of the samples will be done by lamp and wavelength specific to each different element [10].
NFAT5 (TonEBP) mRNA copy number will be assessed by droplet digital PCR (Bio-Rad Laboratories, Segrate, Italy) using the following primers: For: 5′-GAA GTG GAC ATT GAA GGC ACT-3′; Rev: 5′-CTG GCT TCG ACA TCA GCA TT-3′. This technique has already been shown to furnish highly reproducible results as it is based on the absolute count of the gene target abundance, taking advantage of the Poisson statistic that estimates, within the positive droplets (the ones where at least one copy of the target gene is present), the starting copy number of our target gene in terms of copies/µl input sample.
2.6 Potential Confounders
2.6.1 Age and Sex
Consistently with previous animal studies [11], investigation with 23Na-MRI of a cohort entailing 57 patients with HT and 56 normotensive controls [12] reported the skin Na+ to be age- and sex-dependent in all ethnic groups, regardless of the hypertensive or normotensive status, with highest values found in old males. Hence, it seems to be of paramount importance to perform an age- and sex-matched propensity score analysis [13] for these variables (see later).
2.7 Drugs
Considering that antihypertensive drugs, particularly diuretics and blockers of the renin-angiotensin system (RAS), can lower Na+ content in the skin [12], wash-out of possible interfering drugs will be accomplished before studying the patients [14] This will not be applied to the patients diagnosed with PA, who will receive canrenone, for two main reasons: (1) canrenone was shown not to affect the RAS in a prospective study of PA patients [15]; (2) it is necessary to achieve normokalemia and control of the high BP values, e.g. optimal clinical conditions, before the uPA patients are submitted to adrenalectomy[14].
2.8 Sample Size Calculation and Statistical Analysis
Based on the results of a pilot study, which showed a SD of 0.0018 for the Na+/dry weight content in the skin biopsies, we have calculated that 30 surgically treated PA patients and 10 patients with hypertension without PA will be needed to allow detection of a 20% difference between group at a 0.05 alpha (type 1) error with a power of 84% (type II beta error = 16%) using a 2-sided unpaired t-test. Based on the same assumption, we estimated that with use of a paired t-test 15 surgically treated PA patients will be necessary to allow detection of a 20% difference in the Na+/dry weight content in the skin biopsies between baseline and post-surgery, to achieve a statistical power > 95%.
Since this is an observational study prone to the untoward potential effect of confounders on the study endpoints, propensity score matching will be used to minimize the chances of bias when comparing results between uPA and bilPA and between PA patients and PH patients and normotensive patients [13].
3 Discussion
An improvement of knowledge on salt and water in the interstitial space of tissues and organs is by no means a physiological curiosity, but rather a piece of information with implications of paramount importance for organ function in several clinical conditions that feature congestion, including systemic and pulmonary hypertension and heart failure, just to mention a few. For example, induced myocardial congestion was found to be associated with a progressive worsening of pump function at any given preload, in an elegant study in dogs [4], while conversely, marked chronic tissue Na+ depletion in vivo in rats resulted into enhanced contractility in vitro, as assessed by the maximum developed tension of the left ventricle during tetanization in the Langendorff isolated isovolumetric heart [16].
In spite of the importance of tissue edema for organ function, to date, the data documenting changes in skin Na+ in patients with salt-dependent HT are scant: only one study has directly investigated the Na+ content in a sizable cohort of patients with arterial HT [5]. However, it remains altogether uncertain if the reported absence of differences in skin Na+ content between patients with and without HT can be taken for granted, or could just be accounted for by ongoing treatment and/or other confounders owing to the lack of information. Additional studies were performed with 23Na-MRI, which has been suggested to allow a noninvasive quantitative assessment of Na+, both in healthy subjects and in hypertensive patients. According to one such study, tissue Na+ would increase with age and male sex, particularly in the presence of high BP [12]. Other studies of limited series of PA patients suggested an increase in muscle and skin tissue relative Na+ content, which was decreased by treatment with MRA or surgery [17,18,19]. The only double-blind prospective randomized placebo-controlled clinical trial using 23Na-MRI, aimed at assessing the effect of diuretics, including spironolactone, and of low-Na+ diet on muscle and skin Na+, reported intriguing results: after 8 weeks of intervention the skin Na+ content was reported to significantly increase with the low-Na+ diet, and to remain unchanged with diuretics, compared to placebo after adjustment for age, race, sex, BMI and systolic BP [20]. Undoubtedly, 23Na-MRI is a promising and appealing technique, because it lends itself to repeated within-patient studies, owing to its noninvasiveness. However, its accuracy has been validated in animals and in a limited series of amputated legs ex vivo from patients with diabetes or malignancies [19]. Hence, it still needs further validation in human studies in vivo using a gold reference method as the direct measurement of skin Na+, along with K+ and water with atomic absorption spectrophotometry after ashing [21]. Furthermore, parallel 1H-MRI studies are necessary to measure tissue water content [12], and although recently implemented to measure muscle K+ content [22], MRI has not provided information on the skin K+ content in hypertensive patients. Thus, it remains to be settled if, and to what extent, tissue Na+ measurement with 23Na-MRI are affected by concomitant changes of water and other electrolytes.
In this study we will make use of direct chemical-physical analysis, after ashing, to measure electrolytes and water content in skin biopsies, undoubtedly an accurate method, that should be used in future studies as gold standard for referencing 23Na-MRI results.
We believe that this study has some major strengths, including a sizable cohort of patients with a florid form of unilateral surgically curable PA, which should provide enough statistical power, and the direct measurement of the cations and water content in the human skin. The findings concerning the electrolytes and water content might confirm, or perhaps, challenge the classic view of PA being a Na+-dependent form of HT.
Instead of using cure of high BP, which occurs in about 45% of the cases [23] and is affected by several factors, including the long history of HT and the associated presence of vascular remodeling of organ damage, we selected biochemical cure as outcome, because it is the best proxy warranting an unambiguous diagnosis of uPA. At our center, the current rate of biochemical cure after adrenalectomy of uPA patients approximates to 98%. Thus, given this high rate, this study will have a unique opportunity to determine if the detected alterations of Na+, K+, and water content are causally related to hyperaldosteronism and its ongoing treatment with canrenone, or to hypertension per se.
4 Conclusions
In summary, this study will explore the feasibility of repeatedly measuring Na+, K+, and water content in the skin of patients with arterial hypertension, by exploiting careful precautions in collecting the biopsies, measuring electrolytes and water content, and adjusting their values for the specimen dry weight. More importantly, it will provide solid pieces of evidence on cations and water content in the most typical form of human salt-dependent hypertension and on their changes after biochemical cure of hyperaldosteronism with adrenalectomy.
References
Rossier BC. The epithelial sodium channel and the control of blood pressure. J Am Soc Nephrol. 1997;8:980–92.
Campbell NRC, Whelton PK, Orias M, et al. 2022 World Hypertension League, resolve to save lives and International Society of Hypertension dietary sodium (salt) global call to action. J Hum Hypertens. 2023;37:428–37.
Ellison DH, Welling P. Insights into Salt Handling and Blood Pressure. N Engl J Med. 2021;385:1981-1993.
Laine GA, Allen SJ. Left ventricular myocardial edema lymph flow, interstitial fibrosis, and cardiac function. Circ Res [Internet]. 1991;68:1713–1721. Available from: http://ahajournals.org.
Chachaj A, Stanimirova I, Chabowski M, et al. Sodium accumulation in the skin is associated with higher density of skin lymphatic vessels in patients with arterial hypertension. Adv Med Sci. 2023;68:276–89.
Rossi GP, Bisogni V, Bacca AV, et al. The 2020 Italian Society of Arterial Hypertension (SIIA) practical guidelines for the management of primary aldosteronism. Int J Cardiol Hypertens. 2020;5:100029.
Seccia TM, Caroccia B, Gomez-Sanchez EP, et al. The biology of normal zona glomerulosa and aldosterone-producing adenoma: pathological implications. Endocr Rev. 2018;39:1029–56.
Rossitto G, Amar L, Azizi M, et al. Subtyping of primary aldosteronism in the AVIS-2 study: assessment of selectivity and lateralisation. J Clin Endocrinol Metab. 2020;105:2042–52.
Rossi GP, Bisogni V, Rossitto G, et al. Practice Recommendations for Diagnosis and Treatment of the Most Common Forms of Secondary Hypertension. High Blood Press Cardiovasc Prev. 2020;27:547-560.
Sussman IP, MacGregor LC, Masters BR, et al. Quantitative histochemical determination of Na+ and K+ in microscopic samples using carbon furnace atomic absorption spectrometry. J Histochem Cytochem. 1988;36:237–44.
Titze J, Lang R, Ilies C, et al. Osmotically inactive skin Na+ storage in rats. Am J Physiol Renal Physiol. 2003;285:F1108–17.
Kopp C, Linz P, Dahlmann A, et al. 23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension. 2013;61:635–40.
Staffa SJ, Zurakowski D. Five steps to successfully implement and evaluate propensity score matching in clinical research studies. Anesth Analg [Internet]. 2018;127:1066–1073. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29324498.
Rossi GP. Primary aldosteronism: JACC state-of-the-art review [Internet]. J Am Coll Cardiol. 2019:2799–2811. Available from: https://doi.org/10.1016/j.jacc.2019.09.057.
Rossi GP, Ceolotto G, Rossitto G, et al. Effects of Mineralocorticoid and AT1 Receptor Antagonism on The Aldosterone-Renin Ratio In Primary Aldosteronism-the EMIRA Study. J Clin Endocrinol Metab. 2020;105:dgaa080.
Rossi G, Fouad-Tarazi FM. Sodium depletion increases calcium-activated left ventricular pressure in the rat. hypertension [Internet]. 1990;15:894–899. Available from: http://ahajournals.org.
Christa M, Hahner S, Kostler H, et al. Primary hyperaldosteronism induces congruent alterations of sodium homeostasis in different skeletal muscles: a 23Na-MRI study. Eur J Endocrinol. 2022;168:K33–8.
Christa M, Weng AM, Geier B, et al. Increasedmyocardial sodiumsignal intensity in Conn’s syndrome detected by 23Na magnetic resonance imaging. Eur Heart J Cardiovasc Imaging. 2019;20:263–70.
Kopp C, Linz P, Wachsmuth L, et al. 23Na magnetic resonance imaging of tissue sodium. Hypertension. 2012;59:167–72.
Alsouqi A, Deger SM, Sahinoz M, et al. Tissue Sodium in Patients With Early Stage Hypertension: A Randomized Controlled Trial. J Am Heart Assoc. 2022;11:e022723.
Constantinides CD, Kraitchman DL, O’Brien KO, et al. Noninvasive quantification of total sodium concentrations in acute reperfused myocardial infarction using 23Na MRI. Magn Reson Med. 2001;46:1144–51.
Gast LV, Baier LM, Meixner CR, et al. MRI of potassium and sodium enables comprehensive analysis of ion perturbations in skeletal muscle tissue after eccentric exercise. Invest Radiol. 2023;58:265–72.
Rossi GP, Cesari M, Cuspidi C, et al. Long-term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension. 2013;62:62-69.
Maifeld A, Wild J, Karlsen TV, et al. Skin sodium accumulates in psoriasis and reflects disease severity. J Investig Dermatol. 2022;142:166-178.e8.
Braunwald E. Gliflozins in the management of cardiovascular disease. N Engl J Med. 2022;386:2024–34.
Acknowledgements
We acknowledge the support of Dr. Michela Paccagnella and Professor Andrea Mattarei from the Departement of Pharmaceutical and Pharmacological Sciences, University of Padova, without the valuable help and patience of whom, this work would not have been possible to design. We also acknowledge the support of Dr. Giacomo Rossitto for the interpretation of the pilot data and the refinement of protocol.
Funding
Open access funding provided by Università degli Studi di Padova.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Funding
GPR was supported by grants from the FOundation for advanced Research In hypertension and Cardiovascular disease (FORICA).
Compliance with ethical standards
The local Institution review Board and the Ethics committee approved the protocol of this prospective case-control observational study (number of approval: CESC n. 5090/AO/21; URC: AOP1615). All procedures that will be performed in the WHYSKI study are in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Torresan, F., Rossi, F.B., Zanin, S. et al. Water and Electrolyte Content in Salt-Dependent HYpertension in the SKIn (WHYSKI): Effect of Surgical Cure of Primary Aldosteronism. High Blood Press Cardiovasc Prev 31, 15–21 (2024). https://doi.org/10.1007/s40292-023-00614-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40292-023-00614-0