Abstract
Introduction
A number of natural compounds have individually demonstrated to improve glucose and lipid levels in humans.
Aim
To evaluate the short-term glucose and lipid-lowering activity in subjects with impaired fasting glucose.
Methods
To assess the effects of a combination of nutraceuticals based on Lagerstroemia speciosa, Berberis aristata, Curcuma longa, Alpha-lipoic acid, Chrome picolinate and Folic acid, we performed a double-blind, parallel group, placebo-controlled, randomized clinical trial in 40 adults affected by impaired fasting glucose (FPG = 100–125 mg/dL) in primary prevention of cardiovascular disease. After a period of 2 weeks of dietary habits correction only, patients continued the diet and began a period of 8 weeks of treatment with nutraceutical or placebo. Data related to lipid pattern, insulin resistance, liver function and hsCRP were obtained at the baseline and at the end of the study.
Results
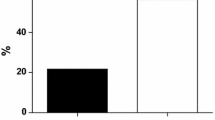
No side effects were detected in both groups of subjects. After the nutraceutical treatment, and compared to the placebo-treated group, the enrolled patients experienced a significant improvement in TG (−34.7%), HDL-C (+13.7), FPI (−13.4%), and HOMA-Index (−25%) versus the baseline values. No significant changes were observed in the other investigated parameters in both groups (Body Mass Index, LDL-C, hsCRP).
Conclusions
The tested combination of nutraceuticals showed clinical efficacy in the improvement of TG, HDL-C, FPI and HOMA-Index, with an optimal tolerability profile. Further confirmation is needed to verify these observations on the middle and long term with a larger number of subjects.
Similar content being viewed by others
1 Introduction
Diabetes is a major social burden, whose prevalence has more than doubled in the last decades [1]. In 2013, the International Diabetes Federation estimated that more than 382 million people are currently affected by diabetes, and forecast that this number will almost double by 2035 [2].
Impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) are pre-diabetic states characterized by elevated glycaemia and associated with insulin resistance and increased cardiovascular risk [3].
The Look AHEAD Trial proved intensive lifestyle intervention to be quite effective in reducing the impact of cardiovascular risk factors in patients with type 2 diabetes mellitus (T2DM) [4], and some studies and meta-analyses showed how physical activity and dietary interventions can lead to a decrease in fasting plasma glucose level (FPG) and prevent or slow down the transition between IFG, IGT and T2DM [3, 5].
Moreover, more than 40 nutraceutical substances have been studied in order to improve lipid and glucose metabolism, and some clinical trials demonstrated a cholesterol-lowering activity, an improvement of glucose metabolism and inflammatory parameters, besides a possible positive influence on cardiovascular prognosis [6].
Berberis aristata (tree turmeric) is a shrub containing the quaternary ammonium salt berberine. Berberine has been demonstrated to improve glucose and lipid metabolism disorders, and to reduce atherogenes is and endothelial inflammation in diabetic patients, with clinical effects similar to those of metformin [7, 8]. Its effect on glucose metabolism, in fact, like metformin, does not depend on insulin concentration [9].
Lagerstroemia speciosa, also known as banaba, is a tropical plant found in the Southeast area of Asia. It has been shown to have, among others, hypoglycaemic and antihyperlipidemic effects, due to corosolic acid and ellagitannins, two of its components [10]. The action mechanisms involved consist of enhanced cellular uptake of glucose, impaired hydrolysis of sucrose and starches, decreased gluconeogenesis and the regulation of lipid metabolism. A supplementation with banaba has proved to be safe and well tolerated in clinical trials [11]. In addition, its action on both glucose and lipid metabolism could be useful in the management of metabolic syndrome [12].
Curcuma longa (turmeric) has long been used in Oriental traditional medicine because of its anti-inflammatory properties. Its main component, curcumin, is responsible foranti-inflammatory, anti-neoplastic, antioxidant and antimicrobial effects [13], with direct therapeutic properties in diabetic subjects [14].
Alpha lipoic acid has an antioxidant effect, with a provenanti-inflammatory and insulin like activity, suggesting a therapeutic role in the management of diabetic and pre diabetic subjects [15].
Chromium picolinate has also proved to be a hypoglycaemic and insulin-resistance reducing substance in patients with T2DM [16].
Folic acid, part of the group B vitamins, has an antioxidant activity. Its supplementation in T2DM patients, together with B12 vitamins, has a positive effect on insulin resistance, fasting plasma glucose level and homocysteinaemia [17].
The association of several nutraceuticals, in particular in patients with two or more risk factors for cardiovascular diseases, has been proved to have a synergistic effect [18, 19].
The aim of this trial is thus to test the effectiveness and safety of a combination of Lagerstroemia speciosa, Berberis aristata, Curcuma longa, Alpha-lipoic acid, Chrome picolinate and Folic acid in subjects with impaired fasting glucose, assessing its short-term effect on glucose and lipid metabolism.
2 Methods
2.1 Study Design
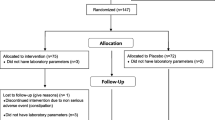
This double-blind, parallel group, placebo-controlled, randomized clinical trial was carried out in 40 adult subjects affected by impaired fasting glucose (FPG = 100–125 mg/dL) in primary prevention of cardiovascular disease, consecutively enrolled in the outpatient service of cardiovascular disease prevention in the Medical and Surgical Sciences Department of the University of Bologna.
The study consisted of a trial with a total duration of 10 weeks. During the first 2 weeks, patients received standard behavioural and qualitative (not quantitative) dietary suggestions to correct unhealthy habits. In particular, subjects were instructed to follow a general indication of a Mediterranean diet, avoiding excessive intake of dairy products and red meat derived products during the study, and maintaining overall constant dietary habits. Individuals were also generically encouraged to increase their physical activity by walking briskly for 20–30 min, 3–5 times per week, or by cycling. After a period of 2 weeks of dietary habits correction only, patients continued the diet and began a period of 8 weeks of treatment with nutraceutical or placebo. Data related to lipid pattern, insulin resistance, liver function and hsCRP were obtained at the baseline and at the end of the study.
The only inclusion criteria was the repeated measure of fasting plasma glucose between 100 and 125 mg/dL.
Exclusion criteria were:
-
Diagnosis of T2DM or assumption of hypoglycaemic drugs/supplement
-
Obesity (BMI >30 kg/m2)
-
Secondary prevention for cardiovascular disease
-
Endocrinological conditions in a failure state (e.g. hypothyroidism, Cushing Syndrome, PCOS)
-
Occasional, programmed or chronic assumption of corticosteroids.
The study was fully conducted in accordance with the Declaration of Helsinki, its protocol was approved by the Ethical Committee of the University of Bologna, and informed consent was obtained from all patients before their inclusion in the study.
2.2 Treatments
After 2 weeks of diet and physical activity stabilization, patients were allocated to treatment with an indistinguishable pill including either placebo or active product containing Lagerstroemia speciosa extract (1% corosolic acid) 250 mg, Berberis aristata extract (98% berberine) 155 mg, curcuma extract 125 mg, chromium picolinate 1.3 mg, folic acid 0.15 mg, solvent-free alpha lipoic acid 110 mg (kindly provided by Pegaso Srl, Arbizzano di Negrar, VR, Italy), two pills per day.
Clinical and laboratory data were obtained at the baseline and after the end of the treatment period.
Randomization was done by assigning an alphabetical code to each batch code (corresponding to treatment or placebo) impressed on the pillbox. Codes were then kept in a sealed envelope, which was not opened until the end of the trial. Pillboxes were then mixed, and the investigators assigned a blinded pillbox to each enrolled patient.
Throughout the study, we instructed patients to take the first dose on the day after they were given the study product. At the same time, all unused products were returned to the investigator.
2.3 Assessments
Personal data, CVD history and pharmaceutical anamnesis of each patient were collected at the beginning of the trial. Anthropometrical parameters, such as body weight, waist circumference (WC) and Body Mass Index (BMI) were collected both at the beginning and during the trial, together with haemodynamic and biochemical parameters. BMI was calculated as weight in kilograms divided by the square of height in metres. Haemodynamic parameters were collected measuring orthostatic and clinostatic systolic and diastolic blood pressure (mean value of three interval measures). Also orthostatic and clinostatic wrist blood pressure (mean value of three interval measures) and cardiac frequency were collected.
All biochemical parameters were obtained after a 12-h overnight fast and The following parameters were obtained or calculated through appropriate formulae: total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C), TG, LDL-C, non-HDL cholesterol (non HDL-C), fasting plasma glucose (FPG), fasting insulin, Serum Uric Acid (SUA), and hsCRP [20]. All measurements were centrally performed in the laboratory of our department with standardized methods by trained biologists [21].
HOMA-IR is the simplest and most commonly used method to estimate insulin resistance. It was calculated as the product of basal glucose (mmol/L) and fasting insulin (μU/mL) divided by 22.5 [22].
Adherence to behavioural counselling and to treatment/placebo, tolerability, acceptability and compliance were also assessed.
2.4 Statistical Analyses
Data were analysed using intention to treat by means of the Statistical Package for Social Science (SPSS) 21.0, version for Windows. Normally distributed baseline characteristics of the population were compared using Student’s t test, non-normally distributed parameters using Mann–Whitney U test. Two-way analyses of variance for crossover design were used to assess the effect of treatment during the assumption of placebo or treatment. All data are expressed as means and standard deviation (SD). To verify the basic assumptions of crossover design, besides the evaluation of period effect, the presence of a carryover effect was excluded. Level of statistical significance was set on p = 0.05.
3 Results
The main characteristics of the enrolled patients are summed up in Table 1.
At the baseline, the two treatment sequence groups were balanced for all the investigated parameters. All the patients completed the study and no one experienced adverse events during the trial.
Compared to baseline values, no change was observed after both treatments as regards WC, BMI, systolic and diastolic blood pressure, total and LDL-cholesterol, and hsCRP (Table 2).
Non HDL-cholesterol and fasting plasma glucose similarly improved in both treated groups (p < 0.05).
Triglycerides were reduced in both groups (p < 0.05), but more in the nutraceutical treated one than in the control group (p < 0.05).
HDL-cholesterol, Fasting insulinaemia and HOMA-index improved in the nutraceutical treated group, only (p < 0.05), when compared both to baseline and to the control group (Tables 2, 3).
4 Discussion
In our trial carried out on subjects with impaired fasting glucose, we observed that a nutraceutical mixture was able to improve on the short term the serum level of TG (−34.7%), HDL-C (+13.7%), FPI (−13.4%), and HOMA-Index (−25%) versus the baseline values (Fig. 1). All these parameters are related to insulin-resistance and metabolic syndrome. Considering that metabolic syndrome is a highly prevalent and incident condition [23] associated with an increased risk of developing type 2 diabetes and cardiovascular disease [24, 25], the improvement of parameters related to the use of the tested product made it a possible interesting therapeutic tool, when associated with a balanced diet. Moreover, the observed results were at least partly expected, because the components of the tested mixture are all supported by an acceptable level of clinical evidence of efficacy [26]. In particular, chlorogenic acid [27], berberine [28], curcumin [29] and chromium picolinate [30] have clearly demonstrated that they exert an insulin-sensitizing effect in humans. On the other hand, the lack of efficacy of the combined nutraceutical on the systemic inflammation evaluated by the measurement of the serum hsCRP is slightly in contrast with what was previously observed with berberine [28] and curcumin [29]: this is probably related to the low doses used of the single nutraceutical components and to the short duration of the treatment. Blood pressure also did non improved beyond the observed improvement of insulin-resistance related parameter, however this could be related to the short duration of the trial and the small improvement on HOMA-index observed.
Then, the tested product was associated with a high tolerability profile that is important to guarantee long-term compliance of the treatment.
Of course, our trial also has some important limitations. First of all the sample size was relatively small, but sufficient to detect the mean FPG difference supposed for the sample size calculation. Then the study was short: its duration was adequate to test the short-term effect, but we are not able to infer our results on the middle-long term. Finally, we did not evaluate an eventual rebound effect on stopping the treatment.
In conclusion, the tested combined nutraceutical was able to improve mildly but significantly several parameters related to insulin-resistance in subjects affected by impaired fasting glucose, both when compared to the baseline and to the control group. Further studies are needed to confirm the effectiveness of this approach to manage insulin-resistant patients on the long term.
References
Zimmet PZ, Magliano DJ, Herman WH, Shaw JE. Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol. 2014;2(1):56–64.
International Diabetes Federation. IDF Diabetes Atlas. 4th ed. Brussels: International Diabetes Federation; 2009.
Gong QH, Kang JF, Ying YY, Li H, Zhang XH, Wu YH, Xu GZ. Lifestyle interventions for adults with impaired glucose tolerance: a systematic review and meta-analysis of the effects on glycemic control. Intern Med. 2015;54(3):303–10.
Johnston CA, Moreno JP, Foreyt JP. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. Curr Atheroscler Rep. 2014;16(12):457.
Yang XJ, Zou SF, Xu Y, Li Y, Yang SS. The influence of intensive lifestyle intervention on patients with isolated impaired fasting glucose: a meta-analysis. J Adv Nurs. 2016;72(11):2587–97.
Cicero AF, Colletti A. Role of phytochemicals in the management of metabolic syndrome. Phytomedicine. 2016;23(11):1134–44.
Caliceti C, Franco P, Spinozzi S, Roda A, Cicero AF. Berberine: new insights from pharmacological aspects to clinical evidences in the management of metabolic disorders. Curr Med Chem. 2016;23(14):1460–76.
Lan J, Zhao Y, Dong F, Yan Z, Zheng W, Fan J, Sun G. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J Ethnopharmacol. 2015;23(161):69–81.
Yin J, Hu R, Chen M, Tang J, Li F, Yang Y, Chen J. Effects of berberine on glucose metabolism in vitro. Metabolism. 2002;51(11):1439–43.
Klein G, Kim J, Himmeldirk K, Cao Y, Chen X. Antidiabetes and anti-obesity activity of Lagerstroemia speciosa. Evid Based Complement Alternat Med. 2007;4(4):401–7.
Stohs SJ, Miller H, Kaats GR. A review of the efficacy and safety of banaba (Lagerstroemia speciosa L.) and corosolic acid. Phytother Res. 2012;26(3):317–24.
Cicero AF, Colletti A. Role of phytochemicals in the management of metabolic syndrome. Phytomedicine. 2016;23(11):1134–44.
Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14(2):141–53.
Meng B, Li J, Cao H. Antioxidant and antiinflammatory activities of curcumin on diabetes mellitus and its complications. Curr Pharm Des. 2013;19(11):2101–13.
Rochette L, Ghibu S, Muresan A, Vergely C. Alpha-lipoic acid: molecular mechanisms and therapeutic potential in diabetes. Can J Physiol Pharmacol. 2015;93(12):1021–7.
Vladeva SV, Terzieva DD, Arabadjiiska DT. Effect of chromium on the insulin resistance in patients with type II diabetes mellitus. Folia Med. 2005;47(3–4):59–62.
Valdés-Ramos R, Guadarrama-López AL, Martínez-Carrillo BE, Benítez-Arciniega AD. Vitamins and type 2 diabetes mellitus. Endocr Metab Immune Disord Drug Targets. 2015;15(1):54–63.
Cicero AF, Colletti A. Combinations of phytomedicines with different lipid lowering activity for dyslipidemia management: the available clinical data. Phytomedicine. 2016;23(11):1113–8.
Volpe R, Sotis G. Nutraceuticals: definition and epidemiological rationale for their use in clinical practice. High Blood Press Cardiovasc Prev. 2015;22(3):199–201.
Cicero AF, Morbini M, Rosticci M, D’Addato S, Grandi E, Borghi C. Middle-term dietary supplementation with red yeast rice plus coenzyme Q10 improves lipid pattern, endothelial reactivity and arterial stiffness in moderately hypercholesterolemic subjects. Ann Nutr Metab. 2016;68(3):213–9.
Cicero AF, Rosticci M, Reggi A, Derosa G, Parini A, Grandi E, D’Addato S, Borghi C. Relationship between serum uric acid and electrocardiographic alterations in a large sample of general population: data from the Brisighella Heart Study. High Blood Press Cardiovasc Prev. 2015;22(2):129–34.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Cicero AF, Nascetti S, Noera G, Gaddi AV, Massa Lombarda Project Team. Metabolic syndrome prevalence in Italy. Nutr Metab Cardiovasc Dis. 2006;16(6):e5–6.
Cicero AF, Derosa G. Are there mild and serious metabolic syndromes? The need for a graded diagnosis. J Cardiovasc Med. 2014;15(10):759–60.
Ambrosioni E, Cicero AF, Parretti D, Filippi A, Rossi A, Peruzzi E, Borghi C. Global cardiovascular disease risk management in Italian patients with metabolic syndrome in the clinical practice setting. High Blood Press Cardiovasc Prev. 2008;15(2):37–45.
Cicero AF, Tartagni E, Ertek S. Nutraceuticals for metabolic syndrome management: from laboratory to benchside. Curr Vasc Pharmacol. 2014;12(4):565–71.
Santana-Gálvez J, Cisneros-Zevallos L, Jacobo-Velázquez DA. Chlorogenic acid: recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules. 2017;22(3). doi: 10.3390/molecules22030358.
Cicero AF, Baggioni A. Berberine and its role in chronic disease. Adv Exp Med Biol. 2016;928:27–45.
Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, Phisalaphong C, Jirawatnotai S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care. 2012;35(11):2121–7.
Ali A, Ma Y, Reynolds J, Wise JP Sr, Inzucchi SE, Katz DL. Chromium effects on glucose tolerance and insulin sensitivity in persons at risk for diabetes mellitus. Endocr Pract. 2011;17(1):16–25.
Acknowledgements
The tested product and the related placebo were kindly furnished by Pegaso Srl, Arbizzano di Negrar, VR, Italy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No one of the authors has a direct conflict of interest in the publication of this paper.
Ethical approval
The trial was approved by the local Ethical Board.
Informed consent
All the enrolled patients signed and informed consent form.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Cicero, A.F.G., Fogacci, F., Morbini, M. et al. Nutraceutical Effects on Glucose and Lipid Metabolism in Patients with Impaired Fasting Glucose: A Pilot, Double-Blind, Placebo-Controlled, Randomized Clinical Trial on a Combined Product. High Blood Press Cardiovasc Prev 24, 283–288 (2017). https://doi.org/10.1007/s40292-017-0206-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40292-017-0206-3