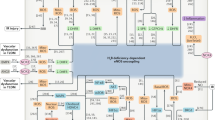
Abstract
NADPH oxidases (NOXs) represent one of the major sources of reactive oxygen species in the vascular district. Reactive oxygen species are responsible for vascular damage that leads to several cardiovascular pathological conditions. Among NOX isoforms, NOX2 is widely expressed in many cells types, such as cardiomyocytes, endothelial cells, and vascular smooth muscle cells, confirming its pivotal role in vascular pathophysiology. Studies in mice models with systemic deletion of NOX2, as well as in transgenic mice overexpressing NOX2, have demonstrated the undeniable involvement of NOX2 in the development of hypertension, atherosclerosis, diabetes mellitus, cardiac hypertrophy, platelet aggregation, and aging. Of note, the inhibition of NOX2 has been found to be protective for cardiovascular homeostasis. Here, we review the evidence demonstrating that the modulation of NOX2 activity is able to improve vascular physiology, suggesting that NOX2 may be a potential target for therapeutic applications.


Similar content being viewed by others
References
Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arter Thromb Vasc Biol. 2005;25:29–38.
Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–4.
Förstermann U. Nitric oxide and oxidative stress in vascular disease. Pflügers Arch. 2010;459:923–39.
Li J-M, Mullen AM, Yun S, et al. Essential role of the NADPH oxidase subunit p47(phox) in endothelial cell superoxide production in response to phorbol ester and tumor necrosis factor-alpha. Circ Res. 2002;90:143–50.
Li JM, Shah AM. Mechanism of endothelial cell NADPH oxidase activation by angiotensin II: role of the p47phox subunit. J Biol Chem. 2003;278:12094–100.
Görlach A, Brandes RP, Nguyen K, et al. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res. 2000;87:26–32.
Ushio-Fukai M, Tang Y, Fukai T, et al. Novel role of gp91phox-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91:1160–7.
Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313.
Meijles DN, Fan LM, Ghazaly MM, et al. p22phox C242T SNP inhibits inflammatory oxidative damage to endothelial cells and vessels. Circulation. 2016;133(24):2391–403.
Carnevale R, Loffredo L, Nocella C, et al. Epicatechin and catechin modulate endothelial activation induced by platelets of patients with peripheral artery disease. Oxid Med Cell Longev. 2014;2014:691015.
Loffredo L, Perri L, Catasca E, et al. Dark chocolate acutely improves walking autonomy in patients with peripheral artery disease. J Am Heart Assoc. 2014;3:e001072.
El Jamali A, Valente AJ, Clark RA. Regulation of phagocyte NADPH oxidase by hydrogen peroxide through a Ca2+/c-Abl signaling pathway. Free Radic Biol Med. 2010;48:798–810.
Dinauer M, Orkin S, Brown R, et al. The glycoprotein encoded by the X-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome b complex. Nature. 1987;717:720.
Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10:427–39.
Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal. 2005;7(3–4):308–17.
Touyz RM, Chen X, Tabet F, et al. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res. 2002;90:1205–13.
Seno T, Inoue N, Gao D, et al. Involvement of NADH/NADPH oxidase in human platelet ROS production. Thromb Res. 2001;103:399–409.
Pignatelli P, Sanguigni V, Lenti L, et al. gp91phox-dependent expression of platelet CD40 ligand. Circulation. 2004;110:1326–9.
Groemping Y, Lapouge K, Smerdon SJ, Rittinger K. Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell. 2003;113:343–55.
Han CH, Freeman JLR, Lee T, et al. Regulation of the neutrophil respiratory burst oxidase: identification of an activation domain in p67(phox). J Biol Chem. 1998;273:16663–8.
Pollock JD, Williams DA, Gifford MA, et al. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–9.
Quie PG, White JG, Holmes B, Good RA. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest. 1967;46:668–79.
Pignatelli P, Carnevale R, Di Santo S, et al. Inherited human gp91phox deficiency is associated with impaired isoprostane formation and platelet dysfunction. Arterioscler Thromb Vasc Biol. 2011;31:423–34.
Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol. 1994;11:95–102.
Jacobson GM, Dourron HM, Liu J, et al. Novel NAD(P)H oxidase inhibitor suppresses angioplasty-induced superoxide and neointimal hyperplasia of rat carotid artery. Circ Res. 2003;92:637–43.
Rajagopalan S, Kurz S, Münzel T, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation: contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–23.
Mollnau H, Wendt M, Szöcs K, et al. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res. 2002;90:E58–65.
Carlström M, Lai EY, Ma Z, et al. Role of NOX2 in the regulation of afferent arteriole responsiveness. Am J Physiol Regul Integr Comp Physiol. 2009;296:R72–9.
Jung O, Schreiber JG, Geiger H, et al. gp91phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation. 2004;109:1795–801.
Murdoch CE, Alom-Ruiz SP, Wang M, et al. Role of endothelial Nox2 NADPH oxidase in angiotensin II-induced hypertension and vasomotor dysfunction. Basic Res Cardiol. 2011;106:527–38.
Bendall JK, Rinze R, Adlam D, et al. Endothelial Nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to angiotensin II: studies in endothelial-targeted Nox2 transgenic mice. Circ Res. 2007;100:1016–25.
Landmesser U, Cai H, Dikalov S, et al. Role of p47phox in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40:511–5.
Touyz RM, Mercure C, He Y, et al. Angiotensin II-dependent chronic hypertension and cardiac hypertrophy are unaffected by gp91phox-containing NADPH oxidase. Hypertension. 2005;45:530–7.
Wang HD, Xu S, Johns DG, et al. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ Res. 2001;88:947–53.
Somers MJ, Mavromatis K, Galis ZS, Harrison DG. Vascular superoxide production and vasomotor function in hypertension induced by deoxycorticosterone acetate-salt. Circulation. 2000;101:1722–8.
Brooks S, Branyan D, Kayla A, et al. Pharmacological inhibition of NAD(P)H oxidase with the peptide gp91ds-tat during ischemic stroke reduces infarct volume and improves vascular reactivity in obese Zucker rats. FASEB J. 2016;30:953–1013.
Girouard H, Park L, Anrather J, et al. Angiotensin II attenuates endothelium-dependent responses in the cerebral microcirculation through nox-2-derived radicals. Arterioscler Thromb. Vasc Biol. 2006;26:826–32.
Chrissobolis S, Banfi B, Sobey CG, Faraci FM. Role of Nox isoforms in angiotensin II-induced oxidative stress and endothelial dysfunction in brain. J Appl Physiol. 2012;113:184–91.
Chan SL, Baumbach GL. Nox2 deficiency prevents hypertension-induced vascular dysfunction and hypertrophy in cerebral arterioles. Int J Hypertens. 2013;2013:793630.
Wind S, Knut B, Armitage ME, et al. Oxidative stress and endothelial dysfunction in aortas of aged spontaneously hypertensive rats by NOX1/2 is reversed by NADPH oxidase inhibition. Hypertension. 2010;56:490–7.
Rey FE, Cifuentes ME, Kiarash, et al. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(-) and systolic blood pressure in mice. Circ Res. 2001;89:408–14.
Sellers KW, Sun C, Diez-Freire C, et al. Novel mechanism of brain soluble epoxide hydrolase-mediated blood pressure regulation in the spontaneously hypertensive rat. FASEB J. 2005;19:626–8.
Zhou X, Bohlen HG, Miller SJ, Unthank JL. NAD(P)H oxidase-derived peroxide mediates elevated basal and impaired flow-induced NO production in SHR mesenteric arteries in vivo. Am J Physiol Heart Circ Physiol. 2008;295:H1008–16.
Zhou MS, Schulman IH, Pagano PJ, et al. Reduced NAD(P)H oxidase in low renin hypertension: link among angiotensin II, atherogenesis, and blood pressure. Hypertension. 2006;47:81–6.
Khan BV, Harrison DG, Olbrych MT, et al. Nitric oxide regulates vascular cell adhesion molecule 1 gene expression and redox-sensitive transcriptional events in human vascular endothelial cells. Proc Natl Acad Sci USA. 1996;93:9114–9.
Sorescu D, Weiss D, Lassegue B, et al. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–35.
Barry-Lane PA, Patterson C, Van Der Merwe M, et al. p47phox is required for atherosclerotic lesion progression in ApoE−/− mice. J Clin Invest. 2001;108:1513–22.
Judkins CP, Diep H, Broughton BRS, et al. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE−/− mice. Am J Physiol Heart Circ Physiol. 2010;298:H24–32.
Kirk EA, Dinauer MC, Rosen H, et al. Impaired superoxide production due to a deficiency in phagocyte NADPH oxidase fails to inhibit atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2000;20:1529–35.
Hsich E, Segal BH, Pagano PJ, et al. Vascular effects following homozygous disruption of p47(phox): an essential component of NADPH oxidase. Circulation. 2000;101:1234–6.
Quesada IM, Lucero A, Amaya C, et al. Selective inactivation of NADPH oxidase 2 causes regression of vascularization and the size and stability of atherosclerotic plaques. Atherosclerosis. 2015;242:469–75.
Dourron HM, Jacobson GM, Park JL, et al. Perivascular gene transfer of NADPH oxidase inhibitor suppresses angioplasty-induced neointimal proliferation of rat carotid artery. Am J Physiol Heart Circ Physiol. 2005;288:H946–53.
Weaver M, Liu J, Pimentel D, et al. Adventitial delivery of dominant-negative p67phox attenuates neointimal hyperplasia of the rat carotid artery. Am J Physiol Heart Circ Physiol. 2006;290:H1933–41.
Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20.
Inoguchi T, Li P, Umeda F, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–45.
Hink U, Li H, Mollnau H, et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:E14–22.
Xiang FL, Lu X, Strutt B, et al. NOX2 deficiency protects against streptozotocin-induced beta-cell destruction and development of diabetes in mice. Diabetes. 2010;59:2603–11.
Zhang M, Ay LK, Anilkumar N, et al. Glycated proteins stimulate reactive oxygen species production in cardiac myocytes: involvement of Nox2 (gp91phox)-containing NADPH oxidase. Circulation. 2006;113:1235–43.
Sukumar P, Viswambharan H, Imrie H, et al. Nox2 NADPH oxidase has a critical role in insulin resistance-related endothelial cell dysfunction. Diabetes. 2013;62:2130–4.
Lynch CM, Kinzenbaw DA, Chen X, et al. Nox2-derived superoxide contributes to cerebral vascular dysfunction in diet-induced obesity. Stroke. 2013;44:3195–201.
Bendall JK, Cave AC, Heymes C, et al. Pivotal role of a gp91phox-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation. 2002;105:293–6.
Satoh M, Ogita H, Takeshita K, et al. Requirement of Rac1 in the development of cardiac hypertrophy. Proc Natl Acad Sci U S A. 2006;103:7432–7.
Häuselmann S, Rosc-Schlüter BI, Lorenz V, et al. b1-Integrin is upregulated via Rac1-dependent reactive oxygen species as part of the hypertrophic cardiomyocyte response. Free Radic Biol Med. 2011;51:609–18.
Tanaka K, Honda M, Takabatake T. Redox regulation of MAPK pathways and cardiac hypertrophy in adult rat cardiac myocyte. J Am Coll Cardiol. 2001;37:676–85.
Patel VB, Bodiga S, Fan D, et al. Cardioprotective effects mediated by angiotensin II type 1 receptor blockade and enhancing angiotensin 1-7 in experimental heart failure in angiotensin-converting enzyme 2-null mice. Hypertension. 2012;59:1195–203.
Byrne JA, Grieve DJ, Bendall JK, et al. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res. 2003;93:802–4.
Grieve DJ, Byrne JA, Siva A, et al. Involvement of the nicotinamide adenosine dinucleotide phosphate oxidase isoform Nox2 in cardiac contractile dysfunction occurring in response to pressure overload. J Am Coll Cardiol. 2006;47:817–26.
Johar S, Cave AC, Narayanapanicker A, et al. Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J. 2006;20:1546–8.
Krijnen PAJ, Meischl C, Hack CE, et al. Increased Nox2 expression in human cardiomyocytes after acute myocardial infarction. J Clin Pathol. 2003;56:194–9.
Fukui T, Yoshiyama M, Hanatani A, et al. Expression of p22-phox and gp91-phox, essential components of NADPH oxidase, increases after myocardial infarction. Biochem Biophys Res Commun. 2001;281:1200–6.
Looi YH, Grieve DJ, Siva A, et al. Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension. 2008;51:319–25.
Zhao Y, McLaughlin D, Robinson E, et al. Nox2 NADPH oxidase promotes pathologic cardiac remodeling associated with doxorubicin chemotherapy. Cancer Res. 2010;70:9287–97.
De Falco E, Roberto C, Pagano F, et al. Role of NOX2 in mediating doxorubicin-induced senescence in human endothelial progenitor cells. Mech Ageing Dev. 2016;159:37–43.
Kahles T, Luedike P, Endres M, et al. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke. 2007;38:3000–6.
Chen H, Song YS, Chan PH. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J Cereb Blood Flow Metab. 2009;29:1262–72.
Brait VH, Jackman KA, Walduck AK, et al. Mechanisms contributing to cerebral infarct size after stroke: gender, reperfusion, T lymphocytes, and Nox2-derived superoxide. J Cereb Blood Flow Metab. 2010;30:1306–17.
Schönbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci. 2001;58:4–43.
Carnevale R, Loffredo L, Sanguigni V, et al. Different degrees of NADPH oxidase 2 regulation and in vivo platelet activation: lesson from chronic granulomatous disease. J Am Heart Assoc. 2014;3(3):e000920.
Carnevale R, Bartimoccia S, Nocella C, et al. LDL oxidation by platelets propagates platelet activation via an oxidative stress-mediated mechanism. Atherosclerosis. 2014;237:108–16.
Walsh TG, Berndt MC, Carrim N, et al. The role of Nox1 and Nox2 in GPVI-dependent platelet activation and thrombus formation. Redox Biol. 2014;2:178–86.
Delaney M, Kim K, Estevez B, et al. Differential roles of the nadph-oxidase 1 and 2 in platelet activation and thrombosis. Arterioscler Thromb Vasc Biol. 2016;36:846–54.
Magwenzi S, Woodward C, Wraith KS, et al. Oxidized LDL activates blood platelets through CD36/NOX2-mediated inhibition of the cGMP/protein kinase G signaling cascade. Blood. 2015;125:2693–703.
Krötz F, Sohn HY, Gloe T, et al. NAD(P)H oxidase-dependent platelet superoxide anion release increases platelet recruitment. Blood. 2002;100:917–24.
Dayal S, Wilson KM, Motto DG, et al. Hydrogen peroxide promotes aging-related platelet hyperactivation and thrombosis. Circulation. 2013;127:1308–16.
Du J, Fan LM, Mai A, Li J-M. Crucial roles of Nox2-derived oxidative stress in deteriorating insulin receptor and endothelial function in dietary obesity of mice after middle age. Br J Pharmacol. 2013;170:1064–77.
Paneni F, Osto E, Costantino S, et al. Deletion of the activated protein-1 transcription factor JunD induces oxidative stress and accelerates age-related endothelial dysfunction. Circulation. 2013;127:1229–40.
Turgeon J, Haddad P, Dussault S, et al. Protection against vascular aging in Nox2-deficient mice: impact on endothelial progenitor cells and reparative neovascularization. Atherosclerosis. 2012;223:122–9.
Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–43.
Barton M. Obesity and aging: determinants of endothelial cell dysfunction and atherosclerosis. Pflugers Arch. 2010;460:825–37.
Meyer MR, Fredette NC, Barton M, Prossnitz ER. Endothelin-1 but not angiotensin II contributes to functional aging in murine carotid arteries. Life Sci. 2014;118:213–8.
Loffredo L, Perri L, Nocella C, Violi F. Antioxidant and antiplatelet activity by polyphenol-rich nutrients: focus on extra-virgin olive oil and cocoa. Br J Clin Pharmacol. 2016. doi:10.1111/bcp.12923.
Carnevale R, Pignatelli P, Nocella C, et al. Extra virgin olive oil blunt post-prandial oxidative stress via NOX2 down-regulation. Atherosclerosis. 2014;235:649–58.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was partially supported by a grant from the Italian Ministry of Health (GR-2013-02355401) to SS and RC.
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
M. Forte and C. Nocella equally contributed to this work.
Rights and permissions
About this article
Cite this article
Forte, M., Nocella, C., De Falco, E. et al. The Pathophysiological Role of NOX2 in Hypertension and Organ Damage. High Blood Press Cardiovasc Prev 23, 355–364 (2016). https://doi.org/10.1007/s40292-016-0175-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40292-016-0175-y