Abstract
Background
Following detection, rupture risk assessment for intracranial aneurysms (IAs) is critical. Towards molecular prognostics, we hypothesized that circulating blood RNA expression profiles are associated with IA risk.
Methods
We performed RNA sequencing on 68 blood samples from IA patients. Here, patients were categorized as either high or low risk by assessment of aneurysm size (≥ 5 mm = high risk) and Population, Hypertension, Age, Size, Earlier subarachnoid hemorrhage, Site (PHASES) score (≥ 1 = high risk). Modified F-statistics and Benjamini-Hochberg false discovery rate correction was performed on transcripts per million-normalized gene counts. Protein-coding genes expressed in ≥ 50% of samples with a q value < 0.05 and an absolute fold-change ≥ 2 were considered significantly differentially expressed. Bioinformatics in Ingenuity Pathway Analysis was performed to understand the biology of risk-associated expression profiles. Association was assessed between gene expression and risk via Pearson correlation analysis. Linear discriminant analysis models using significant genes were created and validated for classification of high-risk cases.
Results
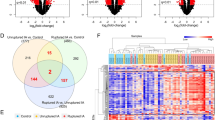
We analyzed transcriptomes of 68 IA patients. In these cases, 31 IAs were large (≥ 5 mm), while 26 IAs had a high PHASES score. Based on size, 36 genes associated with high-risk IAs, and two were correlated with the size measurement. Alternatively, based on PHASES score, 76 genes associated with high-risk cases, and nine of them showed significant correlation to the score. Similar ontological terms were associated with both gene profiles, which reflected inflammatory signaling and vascular remodeling. Prediction models based on size and PHASES stratification were able to correctly predict IA risk status, with > 80% testing accuracy for both.
Conclusions
Here, we identified genes associated with IA risk, as quantified by common clinical metrics. Preliminary classification models demonstrated feasibility of assessing IA risk using whole blood expression.




Similar content being viewed by others
References
Rinkel GJE, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture of intracranial aneurysms. Stroke. 1998;29(1):251–6.
de Rooij NK, Linn FH, van der Plas JA, Algra A, Rinkel GJ. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry. 2007;78(12):1365–72.
Olafsson E, Hauser WA, Gudmundsson G. A population-based study of prognosis of ruptured cerebral aneurysm: mortality and recurrence of subarachnoid hemorrhage. Neurology. 1997;48(5):1191–5.
Bederson JB, Connolly ES Jr, Batjer HH, Dacey RG, Dion JE, Diringer MN, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40(3):994–1025.
Varble N, Tutino-Vincent M, Yu J, Sonig A, Siddiqui-Adnan H, Davies-Jason M, et al. Shared and distinct rupture discriminants of small and large intracranial aneurysms. Stroke. 2018;49(4):856–64.
Wiebers DO. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. The Lancet. 2003;362(9378):103–10.
Greving JP, Wermer MJ, Brown RD Jr, Morita A, Juvela S, Yonekura M, Ishibashi T, Torner JC, Nakayama T, Rinkel GJ, Algra A. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol. 2014;13(1):59–66. https://doi.org/10.1016/S1474-4422(13)70263-1.
Investigators UJ. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012;366(26):2474–82.
Murayama Y, Takao H, Ishibashi T, Saguchi T, Ebara M, Yuki I, et al. Risk analysis of unruptured intracranial aneurysms. Stroke. 2016;47(2):365–71.
Wermer MJH, van der Schaaf IC, Algra A, Rinkel GJE. Risk of rupture of unruptured intracranial aneurysms in relation to patient and aneurysm characteristics. Stroke. 2007;38(4):1404–10.
Tutino VM, Poppenberg KE, Li L, Shallwani H, Jiang K, Jarvis JN, et al. Biomarkers from circulating neutrophil transcriptomes have potential to detect unruptured intracranial aneurysms. J Transl Med. 2018;16(1):373.
Tutino VM, Poppenberg KE, Damiano RJ, Patel TR, Waqas M, Dmytriw AA, et al. Characterization of Long Non-Coding RNA signatures of intracranial aneurysm in circulating whole blood. Mol Diagn Therapy. 2020;24(6):723–36.
Tutino VM, Poppenberg KE, Jiang K, Jarvis JN, Sun Y, Sonig A, et al. Circulating neutrophil transcriptome may reveal intracranial aneurysm signature. PLoS ONE. 2018;13(1): e0191407.
Poppenberg KE, Tutino VM, Li L, Waqas M, June A, Chaves L, et al. Classification models using circulating neutrophil transcripts can detect unruptured intracranial aneurysm. J Transl Med. 2020;18(1):392.
Tutino VM, Zebraski HR, Rajabzadeh-Oghaz H, Waqas M, Jarvis JN, Bach K, et al. Identification of circulating gene expression signatures of intracranial aneurysm in peripheral blood mononuclear cells. Diagnostics. 2021;11:6.
Poppenberg KE, Li L, Waqas M, Paliwal N, Jiang K, Jarvis JN, et al. Whole blood transcriptome biomarkers of unruptured intracranial aneurysm. PLoS ONE. 2020;15(11): e0241838.
Tutino VM, Lu Y, Ishii D, Poppenberg KE, Rajabzadeh-Oghaz H, Siddiqui AH, et al. Aberrant whole blood gene expression in the lumen of human intracranial aneurysms. Diagnostics. 2021;11:8.
Tutino VMFS, Chien A, Patel TR, Monteiro A, Rai HH, Dmytriw AA, Chaves LD, Waqas M, Levy EI, Poppenberg KE, Siddiqui AH. Gene expression profiles of ischemic stroke clots retrieved by mechanical thrombectomy are associated with disease etiology. J NeuroIntervent Surg. 2022;2022:15.
Tutino VM, Fricano S, Frauens K, Patel TR, Monteiro A, Rai HH, et al. Isolation of RNA from acute ischemic stroke clots retrieved by mechanical thrombectomy. Genes. 2021;12:10.
Krämer A, Green J, Pollard J Jr, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinform (Oxf, Engl). 2014;30(4):523–30.
Evans JD. Straightforward statistics for the behavioral sciences. Pacific Grove: Brooks/Cole Pub. Co.; 1996.
Graffeo CS, Tanweer O, Nieves CF, Belmont HM, Izmirly PM, Becske T, et al. Rapid aneurysm growth and rupture in systemic lupus erythematosus. Surg Neurol Int. 2015;6:9.
Lee JH, Lee SW, Choi CH, Ko JK. Does systemic lupus erythematosus increase the risk of procedure-related complication in endovascular treatment of intracranial aneurysm? Yonsei Med J. 2020;61(5):441–4.
Satija R, Farrell JA, Gennert D, Schier AF, Regev A. Spatial reconstruction of single-cell gene expression data. Nat Biotechnol. 2015;33(5):495–502.
McInnes L, Healy J, Melville J. Umap: Uniform manifold approximation and projection for dimension reduction. arXiv:180203426. 2018.
Hussain S, Barbarite E, Chaudhry NS, Gupta K, Dellarole A, Peterson EC, et al. Search for biomarkers of intracranial aneurysms: a systematic review. World Neurosurg. 2015;84(5):1473–83.
Tanriverdi T, Sanus GZ, Ulu MO, Tureci E, Uzun H, Aydin S, et al. Serum and cerebrospinal fluid concentrations of E-selectin in patients with aneurysmal subarachnoid hemorrhage. Braz J Med Biol Res. 2005;38(11):1703–10.
Jia W, Wang R, Zhao J, Liu IY, Zhang D, Wang X, et al. E-Selectin Expression Increased in Human Ruptured Cerebral Aneurysm Tissues. Can J Neurol Sci. 2011;38(6):858–62.
Mack WJ, Mocco J, Hoh DJ, Huang J, Choudhri TF, Kreiter KT, et al. Outcome prediction with serum intercellular adhesion molecule-1 levels after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2002;96(1):71–5.
Witkowska AM, Borawska MH, Socha K, Kochanowicz J, Mariak Z, Konopka M. TNF-α and sICAM-1 in intracranial aneurismal rupture. Arch Immunol Ther Exp. 2009;57(2):137.
Mangieri P, Suzuki K, Ferreira M, Domingues L, Casulari LA. Evaluation of pituitary and thyroid hormones in patients with subarachnoid hemorrhage due to ruptured intracranial aneurysm. Arq Neuropsiquiatr. 2003;61(1):14–9.
Casulari LA, Mangieri P, Naves LA, Suzuki K, Ferreira M, Domingues L. Nonthyroidal illness syndrome in patients with subarachnoid hemorrhage due to intracranial aneurysm. Arq Neuropsiquiatr. 2004;62(1):26–32.
Wiesmann M, Missler U, Hagenström H, Gottmann D. S-100 protein plasma levels after aneurysmal subarachnoid haemorrhage. Acta Neurochirurgica. 1997 1997/12/01;139(12):1155-60.
Ray L, Khemka VK, Behera P, Bandyopadhyay K, Pal S, Pal K, et al. Serum homocysteine, dehydroepiandrosterone sulphate and lipoprotein (a) in alzheimer’s disease and vascular dementia. Aging Dis. 2013;4(2):57–64.
Chen J, Han L, Xu X, Tang H, Wang H, Wei B. Serum biomarkers VEGF-C and IL-6 are associated with severe human Peripheral Artery Stenosis. Journal of inflammation (London, England). 2015;12:50.
Kimura H, Okada O, Tanabe N, Tanaka Y, Terai M, Takiguchi Y, et al. Plasma monocyte chemoattractant protein-1 and pulmonary vascular resistance in chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2001;164(2):319–24.
Korostynski M, Morga R, Piechota M, Hoinkis D, Golda S, Dziedzic T, et al. Inflammatory responses induced by the rupture of intracranial aneurysms are modulated by miRNAs. Mol Neurobiol. 2020;57(2):988–96.
Supriya M, Christopher R, Indira Devi B, Bhat DI, Shukla D. Circulating MicroRNAs as potential molecular biomarkers for intracranial aneurysmal rupture. Mol Diagn Therapy. 2020;24(3):351–64.
Huang Q, Sun Y, Huang Q, Zeng Y, Lin S, Huang S, et al. Association between circular RNAs and intracranial aneurysm rupture under the synergistic effect of individual environmental factors. Front Neurol. 2021;2021:12.
Chalouhi N, Ali MS, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, et al. Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab. 2012;32(9):1659–76.
Tulamo R, Frösen J, Hernesniemi J, Niemelä M. Inflammatory changes in the aneurysm wall: a review. J NeuroIntervent Surg. 2010;2(2):120.
Yokoi T, Saito M, Yoshimura Y, Tsuji K, Nozaki K. Cerebral aneurysms and inflammation. Neuroimmunology and Neuroinflammation. 2015;2:55–8.
Meng H, Tutino VM, Xiang J, Siddiqui A. High WSS or low wss? complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: toward a unifying hypothesis. Am J Neuroradiol. 2014;35(7):1254.
Chyatte D, Bruno G, Desai S, Todor DR. Inflammation and intracranial aneurysms. Neurosurgery. 1999;45(5):1137–47.
Frösen J, Tulamo R, Paetau A, Laaksamo E, Korja M, Laakso A, et al. Saccular intracranial aneurysm: pathology and mechanisms. Acta Neuropathol. 2012;123(6):773–86.
Frösen J, Piippo A, Paetau A, Kangasniemi M, Niemelä M, Hernesniemi J, et al. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture. Stroke. 2004;35(10):2287.
Kataoka K, Taneda M, Asai T, Kinoshita A, Ito M, Kuroda R. Structural fragility and inflammatory response of ruptured cerebral aneurysms. Stroke. 1999;30(7):1396–401.
Tutino VM, Zebraski HR, Rajabzadeh-Oghaz H, Chaves L, Dmytriw AA, Siddiqui AH, et al. RNA sequencing data from human intracranial aneurysm tissue reveals a complex inflammatory environment associated with rupture. Mol Diagn Therapy. 2021;25(6):775–90.
Starke RM, Chalouhi N, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, et al. Critical role of TNF-α in cerebral aneurysm formation and progression to rupture. J Neuroinflamm. 2014;11:77.
Ago T, Sadoshima J. GDF15, a Cardioprotective TGF-β Superfamily Protein. Circul Res. 2006;98(3):294–7.
Kleinloog R, Verweij Bon H, van der Vlies P, Deelen P, Swertz Morris A, de Muynck L, et al. RNA sequencing analysis of intracranial aneurysm walls reveals involvement of lysosomes and immunoglobulins in rupture. Stroke. 2016;47(5):1286–93.
Nakaoka H, Tajima A, Yoneyama T, Hosomichi K, Kasuya H, Mizutani T, et al. Gene expression profiling reveals distinct molecular signatures associated with the rupture of intracranial aneurysm. Stroke. 2014;45(8):2239.
Sharma T, Datta KK, Kumar M, Dey G, Khan AA, Mangalaparthi KK, et al. Intracranial aneurysm biomarker candidates identified by a proteome-wide study. OMICS J Integr Biol. 2020;24(8):483–92.
Field A, Miles J, Field Z. Discovering statistics using R. Thousand Oaks: Sage publications; 2012.
Feghali J, Gami A, Xu R, Jackson CM, Tamargo RJ, McDougall CG, et al. Application of unruptured aneurysm scoring systems to a cohort of ruptured aneurysms: are we underestimating rupture risk? Neurosurg Rev. 2021;44(6):3487–98.
Foreman PM, Hendrix P, Harrigan MR, Fisher WS, Vyas NA, Lipsky RH, et al. PHASES score applied to a prospective cohort of aneurysmal subarachnoid hemorrhage patients. J Clin Neurosci. 2018;53:69–73.
Pagiola I, Mihalea C, Caroff J, Ikka L, Chalumeau V, Iacobucci M, et al. The PHASES score: to treat or not to treat? Retrospective evaluation of the risk of rupture of intracranial aneurysms in patients with aneurysmal subarachnoid hemorrhage. J Neuroradiol. 2020;47(5):349–52.
Neyazi B, Sandalcioglu IE, Maslehaty H. Evaluation of the risk of rupture of intracranial aneurysms in patients with aneurysmal subarachnoid hemorrhage according to the PHASES score. Neurosurg Rev. 2019;42(2):489–92.
Acknowledgements
The authors thank the patients who participated in this study. Special thanks also to Brandon Marzullo, MS, and Jonathan Bard, MS, for RNAseq data analysis assistance, and Jennifer Gay, CCRP, and Elizabeth Bommaraju for study protocol management. This work was performed in part at the New York State Center of Excellence in Bioinformatics and Life Sciences’ Genomics and Bioinformatics Core.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Human Research Institutional Review Board of the University at Buffalo (study number 030474433). Informed consent was obtained from all participants involved in the study, and the study was carried out in accordance with the approved protocols.
Consent for publication
No specific patient information or images are given in the article.
Availability of data and materials
The datasets used in the current study are available from the corresponding author upon reasonable request.
Competing Interests
KEP—None. AC—Awardee of NIH grant, 1R01HL152270-01 and a UCLA Exploratory Research Grant. BAS—None. LC—None. SSV—None. MW—None. AM–None. AAD—None. J-KB—None. MM—None. KVS—Consulting/teaching: Canon Medical Systems Corporation, Penumbra Inc., Medtronic, Jacobs Institute; co-founder: Neurovascular Diagnostics, Inc. AHS—Financial interest/investor/stock options/ownership: Adona Medical, Inc., Amnis Therapeutics, BlinkTBI, Inc., Boston Scientific Corp. (for purchase of Claret Medical), Buffalo Technology Partners, Inc., Cardinal Consultants, LLC, Cerebrotech Medical Systems, Inc., Cognition Medical, Endostream Medical, Ltd, Imperative Care, Inc., International Medical Distribution Partners, Neurovascular Diagnostics, Inc., Q’Apel Medical, Inc., Radical Catheter Technologies, Inc., Rebound Therapeutics Corp. (purchased 2019 by Integra Lifesciences, Corp.), Rist Neurovascular, Inc., Sense Diagnostics, Inc., Serenity Medical, Inc., Silk Road Medical, Spinnaker Medical, Inc., StimMed, Synchron, Three Rivers Medical, Inc., Vastrax, LLC, VICIS, Inc., Viseon, Inc.; consultant/advisory board: Amnis Therapeutics, Boston Scientific, Canon Medical Systems USA, Inc., Cerebrotech Medical Systems, Inc., Cerenovus, Corindus, Inc., Endostream Medical, Ltd, Imperative Care, Inc., Integra LifeSciences Corp., Medtronic, MicroVention, Minnetronix Neuro, Inc., Northwest University – DSMB Chair for HEAT Trial, Penumbra, Q’Apel Medical, Inc., Rapid Medical, Rebound Therapeutics Corp., Serenity Medical, Inc., Silk Road Medical, StimMed, Stryker, Three Rivers Medical, Inc., VasSol, W.L. Gore & Associates; national PI/steering committees: Cerenovus LARGE trial and ARISE II trial, Medtronic SWIFT PRIME and SWIFT DIRECT trials, MicroVention FRED Trial and CONFIDENCE study, MUSC POSITIVE trial, Penumbra 3D Separator trial, COMPASS trial, INVEST trial; research grants: co-investigator, NIH/NINDS 1R01NS091075 Virtual Intervention of Intracranial Aneurysms; role: co-principal investigator, NIH-NINDS R21 NS109575-01 Optimizing Approaches to Endovascular Therapy of Acute Ischemic Stroke. VMT—Principal investigator: National Science Foundation Award No. 1746694 and NIH NINDS award R43 NS115314-0; awardee of a Brain Aneurysm Foundation grant, a Center for Advanced Technology grant, and a Cummings Foundation grant; co-founder: Neurovascular Diagnostics, Inc., QAS.AI, Inc.
Funding
Research reported in this publication was supported, in part, by the Brain Aneurysm Foundation (VMT) and by the National Institute of Neurological Disorders and Stroke of the NIH under award number 1 R43 NS115314-01 to Neurovascular Diagnostics, Inc. (VMT). Additional support was received from the National Heart, Lung, and Blood Institute of the NIH under award number 1R01HL152270-01 (AC) and a UCLA Exploratory Research Grant (AC).
Authors’ contributions
Conceptualization: KEP and VMT; methodology: KEP, BAS, LC, and VMT; software: KEP and VMT; validation: KEP, LC, and VMT; formal analysis: KEP, BAS, LC, SSV, and VMT; investigation: KEP, AHS, and VMT; resources: AHS and VMT; data curation: KEP, MW, AM, J-KB, MM, KVS, AHS, and VMT; writing—original draft preparation: KEP, AC, and VMT; writing—review and editing: all authors; visualization: KEP, SSV, and VMT; supervision: AHS and VMT; project administration: VMT; funding acquisition: VMT. All authors have read and agreed to the published version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Poppenberg, K.E., Chien, A., Santo, B.A. et al. Profiling of Circulating Gene Expression Reveals Molecular Signatures Associated with Intracranial Aneurysm Rupture Risk. Mol Diagn Ther 27, 115–127 (2023). https://doi.org/10.1007/s40291-022-00626-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-022-00626-x