Abstract
Background and Objective
Congenital adrenal hyperplasia involves a series of autosomal recessive disorders where adrenal steroidogenesis is affected. We present a detailed molecular investigation of 13 newborns affected from the severe form of congenital adrenal hyperplasia related to 21-hydroxylase deficiency.
Methods
All patients were diagnosed with classical congenital adrenal hyperplasia in the neonatal period due to adrenal crisis and/or ambiguous genitalia presentation. None of the infants was identified through a congenital adrenal hyperplasia newborn screening program. A molecular analysis of the CYP21A2 gene and a familiar segregation analysis were performed.
Results
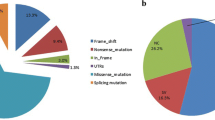
Adrenal crisis was the most severe manifestation in the male salt-wasting newborns while all female patients presented with atypical genitalia. Newborns were correctly genotyped and no genotype-phenotype divergences were found. Two novel severe genotypes, not previously reported, were identified. The novel CYP21A2 frameshift mutations (c.793delG and c.297dupG) were added to the other 45 variants recently reported in the literature, leading to a total count of 279 pathogenic variants affecting the gene.
Conclusions
We have successfully genotyped 13 infants diagnosed with classical congenital adrenal hyperplasia after birth. Our molecular approach led to the identification of two novel frameshift CYP21A2 pathogenic variants related to the salt-wasting form of congenital adrenal hyperplasia.
Similar content being viewed by others
References
Bachelot A, Grouthier V, Courtillot C, Dulon J, Touraine P. Management of endocrine disease: congenital adrenal hyperplasia due to 21-hydroxylase deficiency: update on the management of adult patients and prenatal treatment. Eur J Endocrinol. 2017;176(4):R167–81.
White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev. 2000;21(3):245–91.
Parsa AA, New MI. Steroid 21-hydroxylase deficiency in congenital adrenal hyperplasia. J Steroid Biochem Mol Biol. 2017;165(Pt A):2–11.
Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:4043–88.
Schiffer L, Barnard L, Baranowski ES, Gilligan LC, Taylor AE, Arlt W, et al. Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: a comprehensive review. J Steroid Biochem Mol Biol. 2019;194:105439.
Merke DP, Auchus RJ. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. N Engl J Med. 2020;383(13):1248–61.
Van der Kamp HJ, Noordam K, Elvers B, Van Baarle M, Otten BJ, Verkerk PH. Newborn screening for congenital adrenal hyperplasia in the Netherlands. Pediatrics. 2001;108(6):1320–4.
Gidlof S, Wedell A, Guthenberg C, von Dobeln U, Nordenstrom A. Nationwide neonatal screening for congenital adrenal hyperplasia in Sweden: a 26-year longitudinal prospective population-based study. JAMA Pediatr. 2014;168(6):567–74.
Speiser PW, Dupont B, Rubinstein P, Piazza A, Kastelan A, New MI. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985;37(4):650–67.
Witchel SF. Non-classic congenital adrenal hyperplasia. Steroids. 2013;78(8):747–50.
Bizzarri C, Crea F, Marini R, Benevento D, Porzio O, Rava L, Cappa M. Clinical features suggestive of non-classical 21-hydroxylase deficiency in children presenting with precocious pubarche. J Pediatr Endocrinol Metab. 2012;25(11–12):1059–64.
Nordenstrom A, Falhammar H. Management of endocrine disease: diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. Eur J Endocrinol. 2019;180(3):R127–45.
El-Maouche D, Arlt W, Merke DP. Congenital adrenal hyperplasia. Lancet. 2017;390(10108):2194–210.
Nermoen I, Husebye ES, Myhre AG, Lovas K. Classic congenital adrenal hyperplasia. Tidsskr Nor Laegeforen. 2017;137(7):540–3.
Lee PA, Houk CP, Ahmed SF, Hughes IA, International Consensus Conference on Intersex organized by the Lawson Wilkins Pediatric Endocrine Society, the European Society for Paediatric Endocrinology. Consensus statement on management of intersex disorders. International Consensus on Intersex. Pediatrics. 2006;118(2):e488–500.
Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:364–89.
Falhammar H, Frisen L, Hirschberg AL, Norrby C, Almqvist C, Nordenskjold A, et al. Increased cardiovascular and metabolic morbidity in patients with 21-hydroxylase deficiency: a Swedish population-based national cohort study. J Clin Endocrinol Metab. 2015;100(9):3520–8.
Silveira EL, Elnecave RH, dos Santos EP, Moura V, Pinto EM, van der Linden NI, et al. Molecular analysis of CYP21A2 can optimize the follow-up of positive results in newborn screening for congenital adrenal hyperplasia. Clin Genet. 2009;76:503–10.
Fitness J, Dixit N, Webster D, Torresani T, Pergolizzi R, Speiser PW, et al. Genotyping of CYP21, linked chromosome 6p markers, and a sex-specific gene in neonatal screening for congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1999;84:960–6.
Nordenstrom A, Thilen A, Hagenfeldt L, Larsson A, Wedell A. Genotyping is a valuable diagnostic complement to neonatal screening for congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1999;84(5):1505–9.
Baumgartner-Parzer S, Witsch-Baumgartner M, Hoeppner W. EMQN best practice guidelines for molecular genetic testing and reporting of 21-hydroxylase deficiency. Eur J Hum Genet. 2020;28(10):1341–67.
Concolino P. A rare CYP21A2 haplotype clarifies the phenotype–genotype discrepancy in an Italian patient with non classical congenital adrenal hyperplasia (NC-CAH). Mol Biol Rep. 2020;47(4):3049–52.
Hannah-Shmouni F, Chen W, Merke DP. Genetics of congenital adrenal hyperplasia. Endocrinol Metab Clin N Am. 2017;46(2):435–58.
Concolino P. Issues with the detection of large genomic rearrangements in molecular diagnosis of 21-hydroxylase deficiency. Mol Diagn Ther. 2019;23(5):563–7.
Concolino P, Mello E, Minucci A, Zuppi C, Capoluongo E. Multiplex ligation-dependent probe amplification analysis is useful for diagnosing congenital adrenal hyperplasia but requires a deep knowledge of CYP21A2 genetics. Clin Chem. 2011;57(7):1079–80.
Concolino P, Costella A. Congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency: a comprehensive focus on 233 pathogenic variants of CYP21A2 gene. Mol Diagn Ther. 2018;22(3):261–80.
Simonetti L, Bruque CD, Fernandez CS, Benavides-Mori B, Delea M, Kolomenski JE, et al. CYP21A2 mutation update: comprehensive analysis of databases and published genetic variants. Hum Mutat. 2018;39:5–22.
Carvalho B, Marques CJ, Santos-Silva R, Fontoura M, Carvalho D, Carvalho F. Congenital drenal hyperplasia due to 21-hydroxylase deficiency: an update on genetic analysis of CYP21A2 gene. Exp Clin Endocrinol Diabetes. 2020. https://doi.org/10.1055/a-1108-1419.
New MI, Abraham M, Gonzalez B, Dumic M, Razzaghy-Azar M, Chitayat D, et al. Genotype–phenotype correlation in 1507 families with congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Proc Natl Acad Sci USA. 2013;110:2611–6.
Krone N, Braun A, Roscher AA, Knorr D, Schwarz HP. Predicting phenotype in steroid 21-hydroxylase deficiency? Comprehensive genotyping in 155 unrelated, well defined patients from southern Germany. J Clin Endocrinol Metab. 2000;85(3):1059–65.
Marino R, Ramirez P, Galeano J, Perez Garrido N, Rocco C, Ciaccio M, et al. Steroid 21-hydroxylase gene mutational spectrum in 454 Argentinean patients: genotype–phenotype correlation in a large cohort of patients with congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 2011;75:427–35.
Skordis N, Shammas C, Phedonos AA, Kyriakou A, Toumba M, Neocleous V, et al. Genetic defects of the CYP21A2 gene in girls with premature adrenarche. J Endocrinol Investig. 2015;38:535–9.
Reisch N, Willige M, Kohn D, Schwarz HP, Allolio B, Reincke M, et al. Frequency and causes of adrenal crises over lifetime in patients with 21-hydroxylase deficiency. Eur J Endocrinol. 2012;167:35–42.
Mieszczak J, Houk CP, Lee PA. Assignment of the sex of rearing in the neonate with a disorder of sex development. Curr Opin Pediatr. 2009;21(4):541–7.
Dulin Iniguez E, Ezquieta ZB. Newborn screening of congenital adrenal hyperplasia. Endocrinol Diabetes Nutr. 2018;65(1):1–4.
Chan CL, McFann K, Taylor L, Wright D, Zeitler PS, Barker JM. Congenital adrenal hyperplasia and the second newborn screen. J Pediatr. 2013;163(1):109-13.e1.
Tajima T, Fujikura K, Fukushi M, Hotsubo T, Mitsuhashi Y. Neonatal screening for congenital adrenal hyperplasia in Japan. Pediatr Endocrinol Rev. 2012;10(Suppl. 1):72–8.
Gonzalez EC, Carvajal F, Frometa A, Arteaga AL, Castells EM, Espinosa T, et al. Newborn screening for congenital adrenal hyperplasia in Cuba: six years of experience. Clin Chim Acta. 2013;421:73–8.
Therrell BL Jr, Berenbaum SA, Manter-Kapanke V, Simmank J, Korman K, Prentice L, et al. Results of screening 1.9 million Texas newborns for 21-hydroxylase-deficient congenital adrenal hyperplasia. Pediatrics. 1998;101:583–90.
Pezzuti IL, Barra CB, Mantovani RM, Januario JN, Silva IN. A 3-year follow-up of congenital adrenal hyperplasia newborn screening. J Pediatr (Rio J). 2014;90(3):300–7.
Thil’en A, Nordenstrom A, Hagenfeldt L, von Dobeln U, Guthenberg C, Larsson A. Benefits of neonatal screening for congenital adrenal hyperplasia (21-hydroxylase deficiency) in Sweden. Pediatrics. 1998;101(4):E11.
Heather NL, Seneviratne SN, Webster D, Derraik JG, Jefferies C, Carll J, et al. Newborn screening for congenital adrenal hyperplasia in New Zealand, 1994–2013. J Clin Endocrinol Metab. 2015;100:1002–8.
Witchel SF, Nayak S, Suda-Hartman M, Lee PA. Newborn screening for 21-hydroxylase deficiency: results of CYP21 molecular genetic analysis. J Pediatr. 1997;131(2):328–31.
Minutti CZ, Lacey JM, Magera MJ, Hahn SH, McCann M, Schulze A, et al. Steroid profiling by tandem mass spectrometry improves the positive predictive value of newborn screening for congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2004;89:3687–93.
Falhammar H, Wedell A, Nordenstrom A. Biochemical and genetic diagnosis of 21-hydroxylase deficiency. Endocrine. 2015;50(2):306–14.
van der Linde AAA, Schonbeck Y, van der Kamp HJ, van den Akker ELT, van Albada ME, Boelen A, et al. Evaluation of the Dutch neonatal screening for congenital adrenal hyperplasia. Arch Dis Child. 2019;104:653–7.
Gialluisi A, Menabo S, Baldazzi L, Casula L, Meloni A, Farci MC, et al. A genetic epidemiology study of congenital adrenal hyperplasia in Italy. Clin Genet. 2018;93:223–7.
Senato della Repubblica. Senate Act No. 1756. http://www.senato.it/leg/18/BGT/Schede/Ddliter/52854.htm. Accessed 25 Feb 2021.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, ACMG Laboratory Quality Assurance Committee, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Turan I, Tastan M, Boga DD, Gurbuz F, Kotan LD, Tuli A, et al. 21-Hydroxylase deficiency: mutational spectrum and genotype–phenotype relations analyses by next-generation sequencing and multiplex ligation-dependent probe amplification. Eur J Med Genet. 2020;63:103782.
Gangodkar P, Khadilkar V, Raghupathy P, Kumar R, Dayal AA, Dayal D, et al. Clinical application of a novel next generation sequencing assay for CYP21A2 gene in 310 cases of 21-hydroxylase congenital adrenal hyperplasia from India. Endocrine. 2021;71:189–98. https://doi.org/10.1007/s12020-020-02494-z.
Arteaga E, Valenzuela F, Lagos CF, Lagos M, Martinez A, Baudrand R, et al. Detection of a novel severe mutation affecting the CYP21A2 gene in a Chilean male with salt wasting congenital adrenal hyperplasia. Endocrine. 2020;67:258–63.
Polat S, Karaburgu S, Unluhizarci K, Dundar M, Ozkul Y, Arslan YK, et al. Comprehensive genotyping of Turkish women with hirsutism. J Endocrinol Investig. 2019;42:1077–87.
Dundar A, Bayramov R, Onal MG, Akkus M, Dogan ME, Kenanoglu S, et al. The molecular basis and genotype–phenotype correlations of congenital adrenal hyperplasia (CAH) in Anatolian population. Mol Biol Rep. 2019;46:3677–90.
Yoon JY, Cheon CK. Genotype and clinical outcomes in children with congenital adrenal hyperplasia. Pediatr Int. 2020. https://doi.org/10.1111/ped.14478.
Chi DV, Tran TH, Nguyen DH, Luong LH, Le PT, Ta MH, et al. Novel variants of CYP21A2 in Vietnamese patients with congenital adrenal hyperplasia. Mol Genet Genom Med. 2019;7:e623.
Santos-Silva R, Cardoso R, Lopes L, Fonseca M, Espada F, Sampaio L, On Behalf of the Portuguese Society of Pediatric Endocrinology and Diabetology, et al. CYP21A2 gene pathogenic variants: a multicenter study on genotype–phenotype correlation from a Portuguese pediatric cohort. Horm Res Paediatr. 2019;91:33–45.
Fernandez CS, Taboas M, Bruque CD, Benavides-Mori B, Belli S, Stivel M, et al. Genetic characterization of a large cohort of Argentine 21-hydroxylase deficiency. Clin Endocrinol (Oxf). 2020;93:19–27.
Xu C, Jia W, Cheng X, Ying H, Chen J, Xu J, et al. Genotype–phenotype correlation study and mutational and hormonal analysis in a Chinese cohort with 21-hydroxylase deficiency. Mol Genet Genom Med. 2019;7:e671.
Cohen M, Pignatti E, Dines M, Mory A, Ekhilevitch N, Kolodny R, et al. In silico structural and biochemical functional analysis of a novel CYP21A2 pathogenic variant. Int J Mol Sci. 2020;21(16):5857.
Dayal D, Agarwal M. A novel CYP21A2 gene mutation in classic congenital adrenal hyperplasia. Indian Pediatr. 2019;56:76.
Su L, Yin X, Cheng J, Cai Y, Wu D, Feng Z, et al. Clinical presentation and mutational spectrum in a series of 166 patients with classical 21-hydroxylase deficiency from South China. Clin Chim Acta. 2018;486:142–50.
Karlsson L, de Paula MD, Lusa ALG, D’Almeida Mgnani Silva C, Ostberg LJ, Persson B, et al. Novel non-classic CYP21A2 variants, including combined alleles, identified in patients with congenital adrenal hyperplasia. Clin Biochem. 2019;73:50–6.
Khajuria R, Walia R, Bhansali A, Prasad R. Functional characterization and molecular modeling of the mutations in CYP21A2 gene from patients with congenital adrenal hyperplasia. Biochimie. 2018;149:115–21.
Xu J, Li P. Identification of novel and rare CYP21A2 variants in Chinese patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Clin Biochem. 2019;68:44–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
Paola Concolino and Rosa Maria Paragliola have no conflicts of interest that are directly relevant to the content of this article.
Ethical approval
Ethical review and approval were waived for this study, due to the diagnostic aim of the investigation. All procedures were performed in accordance with the ethical standards of the Ethics Committee of Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome.
informed consent
Informed consent was obtained from all subjects involved in the study.
Availability of Data and Material
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ Contributions
PC and RMP performed the diagnostic investigations and prepared manuscript. All authors have read and approved the final article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Concolino, P., Paragliola, R.M. Molecular Analysis of 21-Hydroxylase Deficiency Reveals Two Novel Severe Genotypes in Affected Newborns. Mol Diagn Ther 25, 327–337 (2021). https://doi.org/10.1007/s40291-021-00520-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-021-00520-y