Abstract
Background and Objective
Bladder cancer is common among current and former smokers. High bladder cancer mortality may be decreased through early diagnosis and screening. The aim of this study was to appraise decision models used for the economic evaluation of bladder cancer screening and diagnosis, and to summarise the main outcomes of these models.
Methods
MEDLINE via PubMed, Embase, EconLit and Web of Science databases was systematically searched from January 2006 to May 2022 for modelling studies that assessed the cost effectiveness of bladder cancer screening and diagnostic interventions. Articles were appraised according to Patient, Intervention, Comparator and Outcome (PICO) characteristics, modelling methods, model structures and data sources. The quality of the studies was also appraised using the Philips checklist by two independent reviewers.
Results
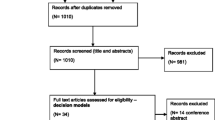
Searches identified 3082 potentially relevant studies, which resulted in 18 articles that met our inclusion criteria. Four of these articles were on bladder cancer screening, and the remaining 14 were diagnostic or surveillance interventions. Two of the four screening models were individual-level simulations. All screening models (n = 4, with three on a high-risk population and one on a general population) concluded that screening is either cost saving or cost effective with cost-effectiveness ratios lower than $53,000/life-years saved. Disease prevalence was a strong determinant of cost effectiveness. Diagnostic models (n = 14) assessed multiple interventions; white light cystoscopy was the most common intervention and was considered cost effective in all studies (n = 4). Screening models relied largely on published evidence generalised from other countries and did not report the validation of their predictions to external data. Almost all diagnostic models (n = 13 out of 14) had a time horizon of 5 years or less and most of the models (n = 11) did not incorporate health-related utilities. In both screening and diagnostic models, epidemiological inputs were based on expert elicitation, assumptions or international evidence of uncertain generalisability. In modelling disease, seven models did not use a standard classification system to define cancer states, others used risk-based, numerical or a Tumour, Node, Metastasis classification. Despite including certain components of disease onset or progression, no models included a complete and coherent model of the natural history of bladder cancer (i.e. simulating the progression of asymptomatic primary bladder cancer from cancer onset, i.e. in the absence of treatment).
Conclusions
The variation in natural history model structures and the lack of data for model parameterisation suggest that research in bladder cancer early detection and screening is at an early stage of development. Appropriate characterisation and analysis of uncertainty in bladder cancer models should be considered a priority.


Similar content being viewed by others
References
Richters A, Aben KKH, Kiemeney LALM. The global burden of urinary bladder cancer: an update. World J Urol. 2020;38(8):1895–904.
Wong MCS, Fung FDH, Leung C, Cheung WWL, Goggins WB, Ng CF. The global epidemiology of bladder cancer: a joinpoint regression analysis of its incidence and mortality trends and projection. Sci Rep. 2018;8(1):1129.
Cumberbatch MGK, Jubber I, Black PC, Esperto F, Figueroa JD, Kamat AM, et al. Epidemiology of bladder cancer: a systematic review and contemporary update of risk factors in 2018. Eur Urol. 2018;74(6):784–95.
Saginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, Barsouk A. Epidemiology of bladder cancer. Med Sci. 2020;8(1):15.
Cumberbatch MG, Rota M, Catto JW, La Vecchia C. The role of tobacco smoke in bladder and kidney carcinogenesis: a comparison of exposures and meta-analysis of incidence and mortality risks. Eur Urol. 2016;70(3):458–66.
Reed O, Jubber I, Griffin J, Noon AP, Goodwin L, Hussain S, et al. Occupational bladder cancer: a cross section survey of previous employments, tasks and exposures matched to cancer phenotypes. PLoS ONE. 2020;15(10): e0239338.
Cumberbatch MG, Cox A, Teare D, Catto JW. Contemporary occupational carcinogen exposure and bladder cancer: a systematic review and meta-analysis. JAMA Oncol. 2015;1(9):1282–90.
Cumberbatch MGK, Noon AP. Epidemiology, aetiology and screening of bladder cancer. Transl Androl Urol. 2019;8(1):5–11.
Jubber I, Shariat SF, Conroy S, Tan WS, Gordon PC, Lotan Y, et al. Non-visible haematuria for the detection of bladder, upper tract, and kidney cancer: an updated systematic review and meta-analysis. Eur Urol. 2020;77(5):583–98.
Kamat AM, Hahn NM, Efstathiou JA, Lerner SP, Malmström P-U, Choi W, et al. Bladder cancer. Lancet. 2016;388(10061):2796–810.
Fujii Y. Prediction models for progression of non-muscle-invasive bladder cancer: a review. Int J Urol. 2018;25(3):212–8.
Larré S, Catto JW, Cookson MS, Messing EM, Shariat SF, Soloway MS, et al. Screening for bladder cancer: rationale, limitations, whom to target, and perspectives. Eur Urol. 2013;63(6):1049–58.
Soria F, Krabbe L-M, Todenhöfer T, Dobruch J, Mitra AP, Inman BA, et al. Molecular markers in bladder cancer. World J Urol. 2019;37(1):31–40.
Linder BJ, Bass EJ, Mostafid H, Boorjian SA. Guideline of guidelines: asymptomatic microscopic haematuria. BJU Int. 2018;121(2):176–83.
Michaeli JC, Boch T, Albers S, Michaeli T, Michaeli DT. Socio-economic burden of disease: survivorship costs for bladder cancer. J Cancer Policy. 2022;32: 100326.
Yeung C, Dinh T, Lee J. The health economics of bladder cancer: an updated review of the published literature. Pharmacoeconomics. 2014;32(11):1093–104.
van Rhijn BWG, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: a systematic review. Eur Urol. 2005;47(6):736–48.
Wang Z, Que H, Suo C, Han Z, Tao J, Huang Z, et al. Evaluation of the NMP22 BladderChek test for detecting bladder cancer: a systematic review and meta-analysis. Oncotarget. 2017;8(59):100648–56.
Gandhi N, Krishna S, Booth CM, Breau RH, Flood TA, Morgan SC, et al. Diagnostic accuracy of magnetic resonance imaging for tumour staging of bladder cancer: systematic review and meta-analysis. BJU Int. 2018;122(5):744–53.
Mandrik O, Severens JL, Bardach A, Ghabri S, Hamel C, Mathes T, et al. Critical appraisal of systematic reviews with costs and cost-effectiveness outcomes: an ISPOR Good Practices Task Force Report. Value Health. 2021;24(4):463–72.
Glanville J, Bayliss S, Booth A, Dundar Y, Fernandes H, Fleeman ND, et al. So many filters, so little time: the development of a search filter appraisal checklist. J Med Libr Assoc. 2008;96(4):356–61.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan: a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210.
Brennan A, Chick SE, Davies R. A taxonomy of model structures for economic evaluation of health technologies. Health Econ. 2006;15(12):1295–310.
Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess. 2004;8(36):iii–iv, ix–xi, 1–158.
Bilcke J, Beutels P, Brisson M, Jit M. Accounting for methodological, structural, and parameter uncertainty in decision-analytic models: a practical guide. Med Decis Mak. 2011;31(4):675–92.
Office for National Statistics. Consumer price inflation tables. 2022. https://www.ons.gov.uk/economy/inflationandpriceindices/datasets/consumerpriceinflation. Accessed 21 Sept 2022.
US Bureau of Labor Statistics. Consumer price index. 2022. https://www.bls.gov/cpi. Accessed 21 Sept 2022.
Organisation for Economic Co-operation and Development. Purchasing power parities (PPP). 2021. https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm. Accessed 21 Sep 2022.
Okubo R, Hoshi SL, Kimura T, Kondo M, Asahi K, Iseki C, et al. Cost-effectiveness of mass screening for dipstick hematuria in Japan. Clin Exp Nephrol. 2022;26(5):398–412.
de Bekker-Grob EW, van der Aa MN, Zwarthoff EC, Eijkemans MJ, van Rhijn BW, van der Kwast TH, et al. Non-muscle-invasive bladder cancer surveillance for which cystoscopy is partly replaced by microsatellite analysis of urine: a cost-effective alternative? BJU Int. 2009;104(1):41–7.
Lotan Y, Svatek RS. Cost-effectiveness of bladder cancer screening. Expert Rev Pharmacoecon Outcomes Res. 2007;7(6):627–32.
Svatek RS, Sagalowsky AI, Lotan Y. Economic impact of screening for bladder cancer using bladder tumor markers: a decision analysis. Urol Oncol. 2006;24(4):338–43.
Sheh KT. An early health economic model of targeted screening for bladder and kidney cancer. In: Sheffield TUO, editor. HAR673 dissertation. The School of Health and Related Research, The University of Sheffield, Sheffield; 2018: p. 79.
Lotan Y, Svatek RS, Sagalowsky AI. Should we screen for bladder cancer in a high-risk population? A cost per life-year saved analysis. Cancer. 2006;107(5):982–90.
Al Hussein Al Awamlh B, Lee R, Chughtai B, Donat SM, Sandhu JS, Herr HW. A cost-effectiveness analysis of management of low-risk non-muscle-invasive bladder cancer using office-based fulguration. Urology. 2015;85(2):381–6.
Garfield SS, Gavaghan MB, Armstrong SO, Jones JS. The cost-effectiveness of blue light cystoscopy in bladder cancer detection: United States projections based on clinical data showing 4.5 years of follow up after a single hexaminolevulinate hydrochloride instillation. Can J Urol. 2013;20(2):6682–9.
Sutton AJ, Lamont JV, Evans RM, Williamson K, O’Rourke D, Duggan B, et al. An early analysis of the cost-effectiveness of a diagnostic classifier for risk stratification of haematuria patients (DCRSHP) compared to flexible cystoscopy in the diagnosis of bladder cancer. PLoS ONE. 2018;13(8): e0202796.
Yuan Z. A partially observable Markov decision process for optimal design of surveillance policies for bladder cancer. In: North Carolina State University, Raleigh, North Carolina. editor. NC; 2012: p. 96.
Mowatt G, Zhu S, Kilonzo M, Boachie C, Fraser C, Griffiths TR, et al. Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer. Health Technol Assess. 2010;14(4):1–331, iii–iv.
Lotan Y, Woldu SL, Sanli O, Black P, Milowsky MI. Modelling cost-effectiveness of a biomarker-based approach to neoadjuvant chemotherapy for muscle-invasive bladder cancer. BJU Int. 2018;122(3):434–40.
Halpern JA, Chughtai B, Ghomrawi H. Cost-effectiveness of common diagnostic approaches for evaluation of asymptomatic microscopic hematuria. JAMA Intern Med. 2017;177(6):800–7.
Rodgers M, Nixon J, Hempel S, Aho T, Kelly J, Neal D, et al. Diagnostic tests and algorithms used in the investigation of haematuria: systematic reviews and economic evaluation. Health Technol Assess. 2006;10(18):iii–iv, xi–259.
Dansk V, Malmström P-U, Bläckberg M, Malmenäs M. Hexaminolevulinate hydrochloride blue-light flexible cystoscopy in the detection and follow-up of nonmuscle-invasive bladder cancer: cost consequences during outpatient surveillance in Sweden. Future Oncol. 2016;12(8):1025–38.
Klaassen Z, Li K, Kassouf W, Black PC, Dragomir A, Kulkarni GS. Contemporary cost-consequence analysis of blue light cystoscopy with hexaminolevulinate in non-muscle-invasive bladder cancer. Can Urol Assoc J. 2017;11(6):173–81.
Rose JB, Armstrong S, Hermann GG, Kjellberg J, Malmström PU. Budget impact of incorporating one instillation of hexaminolevulinate hydrochloride blue-light cytoscopy in transurethral bladder tumour resection for patients with non-muscle-invasive bladder cancer in Sweden. BJU Int. 2016;117(6b):E102–13.
Georgieva MV, Wheeler SB, Erim D, Smith-Bindman R, Loo R, Ng C, et al. Comparison of the harms, advantages, and costs associated with alternative guidelines for the evaluation of hematuria. JAMA Intern Med. 2019;179(10):1352–62.
Mossanen M, Wang Y, Szymaniak J, Tan WS, Huynh MJ, Preston MA, et al. Evaluating the cost of surveillance for non-muscle-invasive bladder cancer: an analysis based on risk categories. World J Urol. 2019;37(10):2059–65.
Kulkarni GS, Alibhai SM, Finelli A, Fleshner NE, Jewett MA, Lopushinsky SR, et al. Cost-effectiveness analysis of immediate radical cystectomy versus intravesical Bacillus Calmette–Guerin therapy for high-risk, high-grade (T1G3) bladder cancer. Cancer. 2009;115(23):5450–9.
Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49(3):466–75 (discussion 75–7).
Kim DD, Basu A. How does cost-effectiveness analysis inform health care decisions? AMA J Ethics. 2021;23(8):E639–47.
Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–28.
Wang SY, Hsu SH, Gross CP, Sanft T, Davidoff AJ, Ma X, et al. Association between time since cancer diagnosis and health-related quality of life: a population-level analysis. Value Health. 2016;19(5):631–8.
Mandrik O, Ekwunife OI, Meheus F, Severens JL, Lhachimi S, Uyl-de Groot CA, et al. Systematic reviews as a “lens of evidence”: determinants of cost-effectiveness of breast cancer screening. Cancer Med. 2019;8(18):7846–58.
Goldie SJ, Kim JJ, Myers E. Chapter 19: cost-effectiveness of cervical cancer screening. Vaccine. 2006;24:S164–70.
Silva-Illanes N, Espinoza M. Critical analysis of Markov models used for the economic evaluation of colorectal cancer screening: a systematic review. Value Health. 2018;21(7):858–73.
Martini A, Sfakianos JP, Renström-Koskela L, Mortezavi A, Falagario UG, Egevad L, et al. The natural history of untreated muscle-invasive bladder cancer. BJU Int. 2020;125(2):270–5.
Drummond M, Barbieri M, Cook J, Glick HA, Lis J, Malik F, et al. Transferability of economic evaluations across jurisdictions: ISPOR good research practices task force report. Value Health. 2009;12(4):409–18.
Daubner-Bendes R, Kovács S, Niewada M, Huic M, Drummond M, Ciani O, et al. Quo vadis HTA for medical devices in central and eastern Europe? Recommendations to address methodological challenges. Front Public Health. 2020;8: 612410.
Gopalappa C, Guo J, Meckoni P, Munkhbat B, Pretorius C, Lauer J, et al. A two-step Markov processes approach for parameterization of cancer state-transition models for low- and middle-income countries. Med Decis Mak. 2018;38(4):520–30.
Schiffer JT, Schiffer CA. To what extent can mathematical modeling inform the design of clinical trials? The example of safe dose reduction of tyrosine kinase inhibitors in responding patients with chronic myeloid leukemia. Haematologica. 2018;103(11):1756–7.
Herzog SA, Blaizot S, Hens N. Mathematical models used to inform study design or surveillance systems in infectious diseases: a systematic review. BMC Infect Dis. 2017;17(1):775.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by the Yorkshire Cancer Research UK (Grant number RA/2019/R1/004). James W.F. Catto is funded by an NIHR Research Professorship.
Conflicts of interest/competing interests
James W.F. Catto reported receiving reimbursement for consultancy from AstraZeneca, Ferring, Roche and Janssen; speaker fees from Bristol Myers Squibb, Merck Sharp & Dohme, Janssen, Astellas, Nucleix and Roche; honoraria for membership in advisory boards from Ferring, Roche, Gilead, Photocure, Bristol Myers Squibb, QED Therapeutics and Janssen; and research funding from Roche. Olena Mandrik, Anne I. Hahn, Ann G. Zauber, Marcus Cumberbatch and James Chilcott have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
The work reported in the paper has been performed by the authors, unless clearly specified in the text. Conceptualization: OM, AIH, AGZ, JC; methodology: OM, JC; analysis and interpretation of data: OM, AIH; drafting of the manuscript: OM, AIH, JWFC, AGZ, MC, JC; funding acquisition: JWFC, JC.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mandrik, O., Hahn, A.I., Catto, J.W.F. et al. Critical Appraisal of Decision Models Used for the Economic Evaluation of Bladder Cancer Screening and Diagnosis: A Systematic Review. PharmacoEconomics 41, 633–650 (2023). https://doi.org/10.1007/s40273-023-01256-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-023-01256-9