Abstract
Objective
Tofacitinib is an oral Janus kinase inhibitor approved for the treatment of ulcerative colitis (UC). The objective of this study was to evaluate the long-term cost-effectiveness of tofacitinib versus current biologics, considering combinations of first-line (1L) and second-line (2L) therapies, from a Japanese payer’s perspective in patients with moderate-to-severe active UC following an inadequate response to conventional therapy and in those who were naïve to biologics.
Methods
A cost-effectiveness analysis was conducted during the time horizon specified in the Markov model, which considers a patient’s lifetime as 60 years and an annual discount rate of 2% on costs and effects. The model compared tofacitinib with vedolizumab, infliximab, adalimumab, golimumab, and ustekinumab. The time of active treatment was divided into induction and maintenance phases. Patients not responding to their biologic treatment after induction or during the maintenance phase were switched to a subsequent line of therapy. Treatment response and remission probabilities (for induction and maintenance phases) were obtained through a systematic literature review and a network meta-analysis that employed a multinomial analysis with fixed effects. Patient characteristics were sourced from the OCTAVE Induction trials. Mean utilities associated with UC health states and adverse events (AEs) were obtained from published sources. Direct medical costs related to drug acquisition, administration, surgery, patient management, and AEs were derived from the JMDC database analysis, which corresponded with the medical procedure fees from 2021. The drug prices were adjusted to April 2021. Further validation through all processes by clinical experts in Japan was conducted to fit the costs to real-world practices. Scenario and sensitivity analyses were also performed to confirm the accuracy and robustness of the base-case results.
Results
In the base-case, the treatment pattern including 1L tofacitinib was more cost-effective than vedolizumab, infliximab, golimumab, and ustekinumab for 1L therapies in terms of cost per quality-adjusted life year (QALY) gained (based on the Japanese threshold of 5,000,000 yen/QALY [38,023 United States dollars {USD}/QALY]). The base-case results demonstrated that the incremental costs would be reduced for all biologics, and decreases in incremental QALYs were observed for all biologics other than adalimumab. The incremental cost-effectiveness ratio (ICER) was found to be dominant for adalimumab; for the other biologics, it was found to be less costly and less efficacious. The efficiency frontier on the cost-effectiveness plane indicated that tofacitinib–infliximab and infliximab–tofacitinib were more cost-effective than the other treatment patterns. When infliximab–tofacitinib was compared with tofacitinib–infliximab, the ICER was 282,609,856 yen/QALY (2,149,157 USD/QALY) and the net monetary benefit (NMB) was −12,741,342 yen (−96,894 USD) with a threshold of 5,000,000 yen (38,023 USD) in Japan. Therefore, infliximab–tofacitinib was not acceptable by this threshold, and tofacitinib–infliximab was the cost-effective treatment pattern.
Conclusion
The current analysis suggests that the treatment pattern including 1L tofacitinib is a cost-effective alternative to the biologics for patients with moderate-to-severe UC from a Japanese payer’s perspective.
Similar content being viewed by others
This study may help make clinical decisions from a cost-effectiveness perspective; it compared the clinical effectiveness and cost-effectiveness of tofacitinib as an alternative treatment option versus currently recommended biologics in Japan (vedolizumab, infliximab, adalimumab, golimumab, and ustekinumab) among moderate-to-severe ulcerative colitis (UC) patients. |
From the Japanese payer's perspective, the analysis of our study suggested that the treatment pattern including first-line tofacitinib is a cost-effective treatment for biologic-naïve patients with moderate-to-severe active UC. |
This study also investigated the relationship between total costs and quality-adjusted life years for all 18 potential treatments, as recommended by clinical guidelines, which may make this study more informative than a simple cost-effective analysis. |
1 Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) characterized by inflammation of the mucosal lining of the colon and rectum [1, 2]. In Japan and other Asian countries, the incidence of UC is rapidly increasing, although it is still lower than in Western countries [3]. A nationwide survey reported that the estimated number of UC patients in Japan was 219,685 in 2014 [4].
The current treatment strategy for UC focuses on clinical response and remission, endoscopic response, and normalization of C-reactive protein/erythrocyte sedimentation rate and calprotectin [5]. The treatments include 5-aminosalicylates, immunomodulators (azathioprine [AZA] or 6-mercaptopurine [6-MP]), and corticosteroids [5, 6]. However, some of the major drawbacks of these drugs include lifelong dependency for mild UC management and ineffectiveness in controlling moderate-to-severe UC [7,8,9], with frequent inconsistent remission and relapse. Moreover, cytapheresis therapy is recommended in Japan and recognized as a safer treatment with minimal side effects [2]. However, cytapheresis is typically only used for patients with disease of moderate severity who can visit medical facilities very often (more than once a week).
The advent of biologics has revolutionized the treatment of UC by targeting key immune system signal modulators involved in inducing and maintaining the state of inflammation and disease severity [10,11,12,13]. Currently, three types of biologics have been approved for the treatment of UC: (1) anti-tumor necrosis factor-α (anti-TNFα) agents (infliximab, adalimumab, and golimumab), (2) anti-integrin agents (vedolizumab), and (3) anti-interleukin-12/23 (anti-IL-12/23) agents (ustekinumab) [10,11,12,13]. These agents have been approved for use either as monotherapy or in combination with certain immunomodulators or conventional/synthetic drugs [13,14,15]. However, the immunogenicity associated with these biologics can lead to secondary non-efficacy, one of the major drawbacks of their use [15,16,17,18,19,20]. In addition, lack of long-term efficacy data (with the exception of infliximab) and relatively high cost are some of the other limitations of biologics for use as treatment of UC [16].
Therefore, to overcome these challenges, a therapy with a novel mechanism of action is being explored for the treatment of UC patients [18, 20,21,22,23]. Tofacitinib is the first oral Janus Kinase inhibitor to be approved in Japan for moderate-to-severe UC [24]. As a small molecule, tofacitinib has many advantages, such as its non-immunogenicity and its suitable and quick absorption, distribution, metabolism, and excretion (ADME) properties, making it an ideal oral formulation [18, 20, 21, 23, 25,26,27]. Double-blinded phase III clinical studies (OCTAVE Induction 1/2 and OCTAVE Sustain) demonstrated that a significantly greater number of patients who received tofacitinib 10 mg twice daily (BID) achieved the primary endpoint of remission at week 8 and week 52 compared to those who received placebo [2, 28,29,30]. In addition, a phase IV clinical trial was completed to establish the effect of tofacitinib in patients with UC in stable remission (NCT03281304) [31].
There are no reports that discuss the efficacy of tofacitinib for UC from a medical-economic point of view in the Japanese environment. Although there is a cost-effectiveness analysis (CEA) model, it is difficult to accurately compare it because it was developed and adopted in countries with different medical insurance and treatment behaviors [32,33,34]. In this study, we modified the existing model to suit Japanese clinical practice and performed a medical-economic analysis of advanced therapies including tofacitinib in UC. The findings from this study may lead to a new treatment decision for patients with moderate-to-severe UC.
2 Methods
2.1 Modeling Approach
An Excel-based CEA model was developed to assess the long-term projections of clinical and cost outcomes from the Japanese payer’s perspective between tofacitinib and all currently recommended biologics to date (vedolizumab, infliximab, adalimumab, golimumab, and ustekinumab) indicated for moderate-to-severe active UC when this study was conducted. Projected outcomes of interest included total costs and quality-adjusted life years (QALYs) gained over a lifelong time horizon (60 years). The average age of Japanese patients was derived from the results of the OCTAVE Induction trials (42.43 years) [28, 30], which indicates the lifetime horizon assumption of 60 years to adequately capture the lifespan of most patients. Cost-effectiveness was described as the incremental cost-effectiveness ratio (ICER), which is the cost per additional unit of QALY gained for the intervention (tofacitinib) versus comparators. In the current analysis, 5,000,000 Japanese yen (JPY) (38,023 United States dollars [USD]) per QALY gained was assumed as a threshold, which is used in Japan for health technology assessments (HTAs) [35]. Both costs and effects were discounted by 2.0% annually for cost-effectiveness evaluation, according to the Japanese cost-effectiveness evaluation guidelines [36], and JPY was adjusted to USD using the annual exchange rate in 2022 (Organization for Economic Co-operation and Development, USD 1 = JPY 131.498) [37].
2.2 Model Structure
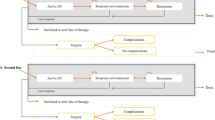
The clinical course and disease progression of UC were described by the Markov model, which is designed to reflect the clinical practices and disease progression of UC in Japan. Each treatment starts with an 8-week induction phase (that is, the 8-week per model cycle length of treatment), the same duration as the OCTAVE Induction trials and consistent with general clinical practices in Japan [28, 30]. Patients who are treatment naïve enter the active UC state in the model and move to a remission or response state according to the efficacy of the treatment (Fig. 1). Patients that do not respond to the treatment at the end of the induction phase will switch to another treatment, while responders continue to stay in the maintenance phase. Patients in the maintenance phase keep the treatment until loss of response (LOR), at which point, they switch to another treatment. Though cytapheresis is occasionally used in Japan, it was not considered as a treatment option in this model, in order to simplify the model. Cytapheresis usually requires frequent visits to medical facilities and sometimes is used in combination with biologics, which may complicate the model. Thus, by not including cytapheresis, we have simplified our model to enable easy interpretation of the results since our study objective was to compare tofacitinib versus biologics for the treatment of moderate-to-severe UC.
Model diagram. Patients who are treatment naïve enter the active UC state in the model and, depending on the efficacy of the treatment, can move to a remission or response state. Patients who do not respond to treatment at the end of induction switch to another treatment, while responders continue to the maintenance phase. Patients in the maintenance phase maintain treatment until loss of response; at that point, they switch to another treatment. At the last health state prior to surgery, the second treatment is continued even if patients were unresponsive to the treatment until their colectomy. Active state UC patients and those who were unresponsive to treatment at any point in the model are at risk of colectomy; patients move to the surgery and post-surgery state. AE adverse event, IPAA ileal pouch-anal anastomosis, UC ulcerative colitis
2.3 Model Validation
The Japanese model was developed based on models validated and published in previous studies [32,33,34]. To adapt to Japanese-specific conditions, the model was based on several assumptions and input parameters that were further validated by three clinical experts (TK, MH, and TH) in Japan (referred to herein as clinical experts). Two of the clinical experts (TK and TH) are Japanese physicians specializing in UC treatment who work in the IBD centers at Japanese core hospitals. The other is a surgeon (MH) belonging to a pharmaceutical company.
2.4 Model Inputs
2.4.1 Patients
Adult patients with moderate-to-severe active UC with an inadequate response, LOR, or intolerance to conventional therapies, such as corticosteroids, AZA, or 6-MP were selected as biologic-naïve patients. The patient characteristics in this model were aligned with the Japanese population being prescribed tofacitinib or placebo (mean age 42.43 years, percentage male 62.90%, weight 59.67 kg, disease duration 8.74 years) in the OCTAVE Induction clinical trial [28, 30].
2.4.2 Treatment Comparators
The model compares tofacitinib to all currently recommended biologics indicated for UC in Japan (Table 1). The doses of treatment were in accordance with the respective drug labels in Japan.
2.4.3 Clinical Efficacy
A systematic literature review (SLR) was conducted to evaluate the efficacy of tofacitinib, biologics, and other treatments for moderate-to-severe UC, based on evidence available from randomized controlled trials (RCTs). A network meta-analysis (NMA) was then conducted to derive estimates of the relative treatment effects between different interventions for patients with moderate-to-severe active UC. The reporting guidelines for Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) were followed throughout the study [38]. The NMA was conducted using the Bayesian framework of OpenBUGS v3.2 (OpenBUGS Foundation) and the R studio version 1.3.1073 software package. A feasibility assessment was conducted for the network and for similarity of patient characteristics in the studies. A fixed effects model was used for each outcome of interest by using the deviance information criterion. The induction and maintenance trials that were analyzed via the NMA are presented in Table 2. The NMA generated transition probabilities between different health states (clinical remission and response) for the induction and maintenance phases in tofacitinib or biologic-naïve patients and tofacitinib or biologic-exposed patients (Table 3). Transition probabilities for the induction phase (8-week per model cycle of treatment) were obtained directly from the NMA results of the induction trials. For vedolizumab, the responses were measured at 6 weeks, obtained from the GEMINI trial [39], and at 10 weeks, obtained from Motoya et al. [40]. It was assumed that the response measurements were comparable. The PRISMA flow diagram of the SLR, details of the NMA, and survival curves based on model simulation are described in the Electronic Supplementary Material (ESM, Figure S1–S10).
Natural mortality can occur at any point in patients during the disease course. We utilized the life tables published by the Ministry of Health, Labour and Welfare in 2018 to take into account the natural mortality [41].
2.4.4 Adverse Events
Risk inputs for adverse events (AEs) in the current model are reported in the ESM Table S1. AEs for analysis included serious infections, upper respiratory tract infections, tuberculosis, malignancies, acute infusion reactions, injection-site reactions, and herpes zoster. As the hazard ratio (HR) for herpes zoster is numerically higher in the Asian population, including Japanese, a higher probability was accounted for by multiplying with the HR reported previously in an RCT [42]. It was further assumed that AEs from tofacitinib or biologics were applied only once at the start of treatment in the induction phase due to the uncertainty of the trial data being used beyond the trial duration. Also, for some treatments, there were no data reported for the stated AE (labeled as “not reported” [NR]).
2.4.5 Treatment Discontinuation
Discontinuation was not explicitly modeled because the comparators’ trials used a non-responder imputation approach to calculate response and remission. Therefore, patients that discontinued trials were counted as non-responders in this study, while the remaining patients continued to receive tofacitinib or biologics as long as they were responding to the treatment. At the last health state prior to surgery, the second treatment was assumed to continue even if patients were unresponsive to the treatment until receiving colectomy.
2.4.6 Colectomy
Patients with active UC who were not responding to the treatments at any point in the model were assumed to be at risk of colectomy. Patients who entered into colectomy were assumed to have received ileal pouch-anal anastomosis, which was identified by the JMDC database as a common surgical procedure in Japan. The JMDC database consists of inpatient, outpatient, dispensing claims, and annual health check-ups from 13 million accumulated patients (as of December 2020) [43]. The database is characterized by its high traceability of insurance enrollees who received treatments in different healthcare institutions [43]. The results of the database analysis were later reviewed and approved by clinical experts. Based on the type of colectomy, early and long-term complications were modeled. The colectomy model inputs and the colectomy complication risks are summarized in the ESM Table S2 and S3.
2.4.7 Utilities
The model estimated health-related quality of life by assigning utilities to health states and disutilities to AEs [44, 45]. A lower utility was applied for the post-colectomy health state. Disutilities associated with AEs from colectomies were not considered as the lower utility assigned to the post-colectomy health state may already account for the disutilities from AEs [46, 47]. Furthermore, the utilities assigned with UC health states (remission, response, and no response) were stratified by the induction and maintenance phases and obtained from the global OCTAVE study involving Japanese patients [28, 30]. Specifically, they were obtained using the EuroQoL 5-dimensions (EQ-5D) questionnaire, and the scores were converted to utilities [48, 49]. In addition, utilities associated with AEs and colectomy were characterized by data derived from non-Japanese patients, as Japanese data for UC patients are limited. The mean utilities for the induction and maintenance phases are reported in Table 4.
2.4.8 Costs
The model captured only direct medical costs (i.e., costs associated with drug acquisition, administration, medical resource use by health/disease status, colectomy, routine resource use, management of AEs, colectomy, and its associated complications); the details are described in ESM Table S4–S11. The dosing regimens for tofacitinib and its comparators were determined based on instructions from their drug labels in Japan. The unit cost of each drug was taken from the list price according to the Japanese National Health Insurance (NHI) (revised in 2021) [50]. Medical resource use and costs regarding drug acquisition and administration, and colectomy were derived from a previous database analysis (hereinafter JMDC database) using NHI costs of April 2020. These medical procedure prices were confirmed as the most recent price from 2021 because there were no price revisions for medical procedures in 2021 [43]. Initially, the list of medical procedures used for UC patients in the JMDC database was developed. A clinical expert then estimated the number of medical resource uses for individual medical procedures associated with UC from their clinical perspective. Moreover, other clinical experts validated their estimated medical resource uses. Finally, all medical resource uses and costs were further reviewed and approved by clinical experts.
2.5 Base-Case Analysis
Tofacitinib is prescribed to moderate-to-severe UC patients who exhibit biologic treatment failure in Japanese clinical practices. Patients receiving tofacitinib as first-line (1L) were transferred to infliximab in second-line (2L) therapy if they experienced primary treatment failure; similarly, patients not on tofacitinib as 1L were transferred to tofacitinib at 2L (Table 5). The assumption to model two lines of treatment was based on claims data in Japan, which indicated patients with UC on biologics or any treatment prior to biologics to be either on a 1L biologic or 2L continuous biologic (53.65% on 1L, 16.04% on 2L) [51]. This assumption was further validated by clinical experts.
2.6 Sensitivity and Scenario Analyses
2.6.1 Multi-way Deterministic Sensitivity Analysis (DSA)
Multi-way DSA is a technique to investigate whether the output of the model is sensitive to the choice of values for each parameter (commonly the key parameters in the model) where more than one parameter in a model is uncertain. During the multi-way DSA, the values of the parameters were changed both individually and as groups in order to assess the impact of each parameter on the model. When standard errors (SEs) were available, input values were individually tested with their respective 95% confidence intervals (CIs), while if SEs were not available, a 20% increase and decrease in the input values were tested. The details can be found in ESM Table S12.
2.6.2 Probabilistic Sensitivity Analysis (PSA)
The parameter uncertainty revolving around cost-effectiveness outcomes was determined by a PSA. In the PSA, the 1000 replications, or repeated simulations, that drew from the distributions of parametric functions, costs, and utility values were conducted. The model predicted the probability of tofacitinib being cost-effective at different thresholds in Japan.
2.6.3 Scenario Analyses
There are multiple treatment patterns for patients with moderate-to-severe UC that are recommended in the guidelines (refer to the American Gastroenterological Association [52], American College of Gastroenterology [53, 54], American Society of Colon and Rectal Surgeons [55], and European Crohn's and Colitis Organization [56]). Therefore, treatments are dependent on the preferences of physicians and the drugs available in their hospitals. As a scenario analysis, we compared 18 treatment patterns recommended in the above guidelines that were validated by clinical experts (ESM Table S13). Infliximab biosimilar is included in the treatment patterns as a representative drug for TNFα biosimilars. Strictly speaking, the efficacy of a biosimilar is different from original drugs; however, the efficacy and transition probability in the model were set as equivalent to the original infliximab values, while the drug cost of infliximab was changed (36,980 yen/vial [281 USD/vial]) (ESM Table S4).
For the exploratory scenario analysis, in order to account for the LOR and increasing dose, a scenario with exposed patients in the maintenance period receiving tofacitinib at 10 mg BID (up from 5 mg BID in the base-case analysis) was conducted. It was assumed that the total cost of tofacitinib would be higher because of the higher dose; moreover, the change in response and remission was taken into account from the regular dosage of 5 mg BID (transition probabilities: 36.41% and 15.65%, respectively).
3 Results
3.1 Base-Case Analysis
The base-case results demonstrated that the incremental costs would be reduced for all biologics: vedolizumab–tofacitinib, −13,861,346 yen (−105,411 USD); infliximab–tofacitinib, −12,970,825 yen (−98,639 USD); adalimumab–tofacitinib, −13,729,437 yen (−104,408 USD); golimumab–tofacitinib, −13,663,320 yen (−103,905 USD); ustekinumab–tofacitinib, −15,726,316 yen (−119,594 USD); see Table 6. In addition, decreases in incremental QALYs were observed for all biologics other than adalimumab, which showed an increase of 0.023 QALY. Finally, while the ICER was found to be dominant for adalimumab, for the other biologics, it was found to be less costly but also had lower QALYs.
The efficiency frontier on the cost-effectiveness plane indicated that tofacitinib–infliximab and infliximab–tofacitinib were more cost-effective than the other treatment patterns (Fig. 2). When infliximab–tofacitinib was compared with tofacitinib–infliximab, the ICER was 282,609,856 yen/QALY (2,149,157 USD/QALY) and the net monetary benefit (NMB) was −12,741,342 yen (−96,894 USD) with a threshold of 5,000,000 yen (38,023 USD). Therefore, infliximab–tofacitinib was not acceptable by this threshold, and tofacitinib–infliximab was the cost-effective treatment pattern.
3.2 Multi-way DSA
A multi-way DSA was conducted by changing the parameters (either through adjusting the 95% CI or by a 20% increase and decrease of the input value) to test for multiple scenarios (ESM Table S12). The 5- and 10-year time horizon scenarios were consistent with the base-case results. Moreover, almost none of the scenarios differed significantly from the base-case results.
3.3 PSA
The incremental costs and QALY results between tofacitinib and each biologic by replication, as presented in the scatterplot, suggest a wide range in expected incremental QALYs (Fig. 3). The cost-effectiveness acceptability curve (CEAC) shows the probability of tofacitinib–infliximab being cost-effective at different thresholds in Japan when compared across all comparators (Fig. 4). These results indicate that tofacitinib–infliximab is acceptable in terms of cost-effectiveness with any threshold between 0 and 10,000,000 yen/QALY (0–76047 USD/QALY).
3.4 Scenario Analyses
Figure 5 shows the relationship between total costs and QALYs for all 18 potential treatments, as recommended by clinical guidelines. From the efficiency frontier plot, which illustrates the QALY that can be accomplished for the cost, the combinations that lie along this efficiency frontier are cost-effective in this plot. Tofacitinib–infliximab biosimilar, vedolizumab–infliximab biosimilar, and infliximab biosimilar–tofacitinib are on the frontier line indicating their relative efficiency over the other treatment patterns. The ICER and NMB values for these three treatment patterns are as follows (ESM Table S14):
-
Tofacitinib–infliximab biosimilar versus vedolizumab–infliximab biosimilar had an ICER of 13,370,825 yen (101,681 USD) and an NMB of −90,524 yen (−688 USD).
-
Tofacitinib–infliximab biosimilar versus infliximab biosimilar–tofacitinib had an ICER of 640,693,617 yen (4,872,269 USD) and an NMB of −29,176,160 yen (−221,875 USD).
These results indicate that the tofacitinib–infliximab biosimilar treatment pattern is cost-effective.
Increasing the dosage of tofacitinib from 5 to 10 mg during the maintenance period resulted in vedolizumab–infliximab biosimilar, infliximab biosimilar–vedolizumab, and infliximab biosimilar–facitinib being on the efficiency frontier indicating that these treatment patterns were cost-effective over the other treatment patterns. The ICER and NMB numbers for these three treatment patterns are as follows (ESM Table S15):
-
Vedolizumab–infliximab biosimilar versus infliximab biosimilar–vedolizumab had an ICER of 1,985,924,503 yen (15,102,317 USD) and an NMB of −26,706,243 yen (−203,092 USD).
-
Vedolizumab–infliximab biosimilar versus infliximab biosimilar–tofacitinib had an ICER 2,168,945,631 yen (16,494,134 USD) and an NMB of −80,448,924 yen (−611,788 USD).
These results indicate that the vedolizumab–infliximab biosimilar treatment pattern is cost-effective.
4 Discussion
To the best of our knowledge, this is the first study that focuses on a Japanese clinical setting and lifetime horizon to compare tofacitinib to biologics (vedolizumab, infliximab, adalimumab, golimumab, and ustekinumab) among moderate-to-severe UC patients.
As there are no prior studies comparing CEA of tofacitinib for UC in Japan, a Japanese-specific Markov simulation model was built to predict where, in the current Japanese treatment paradigm, tofacitinib might provide the optimal clinical benefit (in terms of QALYs) for patients with moderate-to-severe active UC. The current model demonstrated that the treatment pattern including 1L tofacitinib seems to be the cost-effective therapy over biologic comparators at a threshold of 5,000,000 yen (38,023 USD).
A study from Wu et al. [33], like our study, compared treatment patterns on a cost-effectiveness plane. In the Wu et al. study, clinical remission and response were not calculated separately for the treatment-naïve and exposed populations. The study thus assumed that the effect of the 2L treatment was the same as that in the treatment-naïve population even in patients who had failed 1L treatments. On the other hand, in our study, clinical remission and response are calculated for both the naïve and exposed populations, which allowed us to conduct a more detailed analysis on cost-effectiveness. Furthermore, in the Wu et al. study, with the exception of tofacitinib, the clinical remission and response of the maintenance phase of vedolizumab are higher than other treatments, contributing to the higher QALYs of treatment patterns including vedolizumab and tofacitinib. In 18 treatment patterns of the scenario analysis in our study, the QALYs of the treatment patterns including tofacitinib (which have a high success rate of treatment-exposed patients entering clinical remission) are high, so that the structure of the model allows for a longer period for the 2L treatment.
In another study, Taxonera et al. [57] analyzed treatment patterns of tofacitinib–infliximab and vedolizumab–infliximab in a biologic-naïve population [57]. Like our study, patients were modeled to continue the 2L treatment until either surgical intervention or death. In the base case of Taxonera et al., tofacitinib–infliximab is less costly than vedolizumab–infliximab by €23,815.58, and there was only a small positive difference in total QALYs (0.00014). On the other hand, in their scenario analysis, the ICER results were not robust, as demonstrated by ICER decreasing in cost and efficiency when the time horizon and annual surgery risks were changed. Our scenario analysis comparing 18 treatment patterns reports similar results in that our model found the QALY of tofacitinib–infliximab biosimilar was lower than that of vedolizumab–infliximab biosimilar. In the base-case analysis of our study, life years (LYs) did not differ between treatment patterns, and Taxonera et al. indeed reported an LY difference of just −0.0000046. Since tofacitinib and TNFα are not drugs that directly affect mortality, there may be very little difference in LYs and therefore very little difference in QALYs. Therefore, the study from Taxonera et al. used a similar model and population to our study, and our results were generally in agreement, indicating the robustness of our results.
Increasing the dose of tofacitinib from 5 to 10 mg during the maintenance period resulted in vedolizumab–infliximab biosimilar, infliximab biosimilar–vedolizumab, and infliximab biosimilar–tofacitinib on the efficiency frontier, which differed from the frontier from a dose of 5 mg. However, vedolizumab–infliximab biosimilar and infliximab biosimilar–tofacitinib were on the frontier in both analyses. Therefore, both treatment patterns were more cost-effective than the other treatment patterns.
As with any modeling analysis, this study also has limitations that need to be considered for proper interpretation of the results. First, several assumptions such as the modeling approach for 2L therapy continuation, transition probability of vedolizumab, and the medical resource usage for cost parameters had to be made to account for missing data to perform the analysis. These assumptions were, however, validated by clinical experts and health economists in Japan and were not shown to be the major drivers of cost-effective results in our study. Second, our model structure utilized a fixed 8-week induction length for all treatments and did not account for variable induction lengths among treatments. While this approach is aligned with other published models [33] and health technology appraisals [58, 59], we recognize it as a potential limitation to the model structure, and there may be a difference between this approach and clinical practice. Third, our model did not capture dose optimization for the biologic comparators due to limited information to define changes in dose and/or administration, although dose escalation has not yet been approved in Japan, except for adalimumab. However, we compared two patterns of scenario analyses: the first compared 18 treatment patterns by tofacitinib 5 mg and the second compared 18 treatment patterns by dose variation of tofacitinib from the regular 5–10 mg BID. In both scenario analyses, vedolizumab–infliximab biosimilar and infliximab biosimilar–tofacitinib were on the frontier and seemed to be more cost-effective than the other treatment patterns. Fourth, we did not consider cytapheresis therapy as a potential treatment in the model. Fifth, we did not conduct comprehensive scenario analysis with all combination patterns for 1L and 2L therapy due to prioritization of the patterns based on the guideline recommendations [52, 53, 55, 56]. Sixth, because of the absence of suitable quality-of-life studies in Japan, Japanese-specific utility data for AEs and colectomy were not available, and consequently, non-Japanese data had to be inputted. Seventh, our objective was to observe biologic-naïve patients, and therefore, we did not use a mixed population, to avoid the complexities from analyses, for simple interpretation of the results. Eighth, we targeted biologics recommended by the UC treatment guidelines in Japan for 2021. Recently, filgotinib and upadacitinib were launched for UC treatment in Japan, though they have not been recommended by the Japanese guidelines yet. However, CEA including filgotinib and upadacitinib will be needed in the near future once they have been recognized in Japan as standards for UC treatment. Ninth, it is known that tofacitinib is associated with serious heart-related events like stroke and heart attack. However, we considered AEs frequently observed during clinical trials in the model, as well as in the previous studies [32,33,34]. Tenth, we did not consider discontinuation due to AEs, but the rate of no response to treatments was considered in the model. If patients do not respond to the treatment (including those who discontinue due to AEs), they receive the next treatment in the model. Therefore, the discontinuation of treatments due to AEs is indirectly considered.
Most importantly, given the absence of efficacy data in direct comparison between the two alternatives, it was imperative to perform indirect comparisons using an NMA. However, there is a current data gap in estimating the clinical efficacy of some biologics through these indirect comparisons [34]. As in our analysis, indirect comparisons could not be made for infliximab and golimumab in patients that were exposed to biologics due to the limited data available from RCTs. Missing efficacy was estimated by calculating the relative risk in efficacy between the two subpopulations (naïve to and exposed to biologics) for other biologics with data. The relative risk was then applied to the efficacy of infliximab and golimumab in patients naïve to biologics to calculate the efficacy in patients exposed to biologics. Additionally, the GEMINI trial [39] (vedolizumab) employed re-randomization in the maintenance phase, which made evaluation of efficacy in the same network as other biologics (treat-through design) difficult. Therefore, we utilized the NMA for the studies with a re-randomized assignment design and calculated the relative risk for vedolizumab compared to placebo from a re-randomized NMA. Relative risks were then applied to the treat-through probabilities of placebo to estimate the probabilities of vedolizumab.
Notwithstanding these limitations, our model could follow patients over a lifetime horizon and until their death, as opposed to prior models with shorter time horizons (5–10 years) [60, 61]. This was a better reflection of treatment patterns in UC. Finally, our economic model was based on the latest and best available evidence, resulting in reliable results, the robustness of which were demonstrated by the sensitivity analysis conducted as part of our study.
5 Conclusion
Health economic evaluations for rational drug use should be encouraged, as they provide meaningful information on the cost-effectiveness of alternative treatment patterns over canonical treatments. Our study suggested that the treatment pattern including 1L tofacitinib is a cost-effective treatment for biologic-naïve patients with moderate-to-severe active UC and is likely to be cost-effective compared with biologics from the Japanese payer’s perspective.
References
Kobayashi T, Siegmund B, Le Berre C, Wei SC, Ferrante M, Shen B, et al. Ulcerative colitis. Nat Rev Dis Primers. 2020;6(1):74. https://doi.org/10.1038/s41572-020-0205-x.
Nakase H, Uchino M, Shinzaki S, Matsuura M, Matsuoka K, Kobayashi T, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. 2021;56(6):489–526. https://doi.org/10.1007/s00535-021-01784-1.
Hammer T, Langholz E. The epidemiology of inflammatory bowel disease: balance between East and West? A narrative review. Dig Med Res. 2020;3:48.
Murakami Y, Nishiwaki Y, Oba MS, Asakura K, Ohfuji S, Fukushima W, et al. Correction to: Estimated prevalence of ulcerative colitis and Crohn’s disease in Japan in 2015: an analysis of a nationwide survey. J Gastroenterol. 2020;55(1):131. https://doi.org/10.1007/s00535-019-01637-y.
Turner D, Ricciuto A, Lewis A, D’Amico F, Dhaliwal J, Griffiths AM, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570–83. https://doi.org/10.1053/j.gastro.2020.12.031.
Nakase H. Optimizing the use of current treatments and emerging therapeutic approaches to achieve therapeutic success in patients with inflammatory bowel disease. Gut Liver. 2020;14(1):7–19. https://doi.org/10.5009/gnl18203.
Safety and Efficacy of QBECO in Moderate to Severe Ulcerative Colitis. https://clinicaltrials.gov/ct2/show/results/NCT02426372?view=results.
MAYO SCORE FOR ASSESSMENT OF ULCERATIVE COLITIS—UPDATED. https://globalrph.com/medcalcs/mayo-score-for-assessment-of-ulcerative-colitis-updated/.
Pabla BS, Schwartz DA. Assessing severity of disease in patients with ulcerative colitis. Gastroenterol Clin N Am. 2020;49(4):671–88. https://doi.org/10.1016/j.gtc.2020.08.003.
Chao YS, Loshak H. CADTH Rapid Response Reports. Biologics versus Immunomodulators for the Treatment of Ulcerative Colitis: a Review of Comparative Clinical Effectiveness and Cost-Effectiveness. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health Copyright © 2019 Canadian Agency for Drugs and Technologies in Health. 2019.
Arora Z, Shen B. Biological therapy for ulcerative colitis. Gastroenterol Rep (Oxf). 2015;3(2):103–9. https://doi.org/10.1093/gastro/gou070.
Bhattacharya A, Osterman MT. Biologic therapy for ulcerative colitis. Gastroenterol Clin N Am. 2020;49(4):717–29. https://doi.org/10.1016/j.gtc.2020.08.002.
Breton J, Kastl A, Conrad MA, Baldassano RN. Positioning biologic therapies in the management of pediatric inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2020;16(8):400–14.
Lichtenstein GR, Diamond RH, Wagner CL, Fasanmade AA, Olson AD, Marano CW, et al. Clinical trial: benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment Pharmacol Ther. 2009;30(3):210–26. https://doi.org/10.1111/j.1365-2036.2009.04027.x.
Gold SL, Steinlauf AF. Efficacy and safety of dual biologic therapy in patients with inflammatory bowel disease: a review of the literature. Gastroenterol Hepatol (N Y). 2021;17(9):406–14.
The Pros and Cons of Biologics for Ulcerative Colitis. https://www.everydayhealth.com/ulcerative-colitis/treatment/pros-and-cons-biologics-ulcerative-colitis/.
Battle of the Biologics in Ulcerative Colitis. https://www.medpagetoday.com/gastroenterology/inflammatoryboweldisease/84463.
Massalska M, Maslinski W, Ciechomska M. Small molecule inhibitors in the treatment of rheumatoid arthritis and beyond: latest updates and potential strategy for fighting COVID-19. Cells. 2020. https://doi.org/10.3390/cells9081876.
Bonovas S, Pantavou K, Evripidou D, Bastiampillai AJ, Nikolopoulos GK, Peyrin-Biroulet L, et al. Safety of biological therapies in ulcerative colitis: an umbrella review of meta-analyses. Best Pract Res Clin Gastroenterol. 2018;32–33:43–7. https://doi.org/10.1016/j.bpg.2018.05.005.
Bonovas S, Lytras T, Nikolopoulos G, Peyrin-Biroulet L, Danese S. Systematic review with network meta-analysis: comparative assessment of tofacitinib and biological therapies for moderate-to-severe ulcerative colitis. Aliment Pharmacol Ther. 2018;47(4):454–65. https://doi.org/10.1111/apt.14449.
Ferrante M, Sabino J. Efficacy of JAK inhibitors in ulcerative colitis. J Crohns Colitis. 2020;14(Supplement_2):S737–45. https://doi.org/10.1093/ecco-jcc/jjz202.
Cordes F, Foell D, Ding JN, Varga G, Bettenworth D. Differential regulation of JAK/STAT-signaling in patients with ulcerative colitis and Crohn’s disease. World J Gastroenterol. 2020;26(28):4055–75. https://doi.org/10.3748/wjg.v26.i28.4055.
Sandborn WJ, Peyrin-Biroulet L, Sharara AI, Su C, Modesto I, Mundayat R, et al. Efficacy and safety of tofacitinib in ulcerative colitis based on prior tumor necrosis factor inhibitor failure status. Clin Gastroenterol Hepatol. 2022;20(3):591-601.e8. https://doi.org/10.1016/j.cgh.2021.02.043.
Pharmaceutical Evaluation Division, Pharmaceutical Safety and Environmental Health Bureau Ministry of Health, Labour and Welfare https://www.pmda.go.jp/files/000237584.pdf.
D’Amico F, Parigi TL, Fiorino G, Peyrin-Biroulet L, Danese S. Tofacitinib in the treatment of ulcerative colitis: efficacy and safety from clinical trials to real-world experience. Therap Adv Gastroenterol. 2019;12:1756284819848631. https://doi.org/10.1177/1756284819848631.
Lohan C, Diamantopoulos A, LeReun C, Wright E, Bohm N, Sawyer LM. Tofacitinib for the treatment of moderately to severely active ulcerative colitis: a systematic review, network meta-analysis and economic evaluation. BMJ Open Gastroenterol. 2019;6(1):e000302. https://doi.org/10.1136/bmjgast-2019-000302.
Fernández-Clotet A, Castro-Poceiro J, Panés J. Tofacitinib for the treatment of ulcerative colitis. Expert Rev Clin Immunol. 2018;14(11):881–92. https://doi.org/10.1080/1744666x.2018.1532291.
Sandborn WJ, Su C, Sands BE, D’Haens GR, Vermeire S, Schreiber S, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376(18):1723–36. https://doi.org/10.1056/NEJMoa1606910.
Panés J, Vermeire S, Dubinsky MC, Loftus EV, Lawendy N, Wang W, et al. Efficacy and safety of tofacitinib re-treatment for ulcerative colitis after treatment interruption: results from the OCTAVE clinical trials. J Crohns Colitis. 2021;15(11):1852–63. https://doi.org/10.1093/ecco-jcc/jjab065.
Sandborn WJ, Peyrin-Biroulet L, Quirk D, Wang W, Nduaka CI, Mukherjee A, et al. Efficacy and safety of extended induction with tofacitinib for the treatment of ulcerative colitis. Clin Gastroenterol Hepatol. 2020. https://doi.org/10.1016/j.cgh.2020.10.038.
A study of tofacitinib in patients with ulcerative colitis in stable remission. https://clinicaltrials.gov/ct2/show/NCT03281304?term=Tofacitinib&cond=Ulcerative+Colitis&cntry=JP&draw=2&rank=3.
Milev S, DiBonaventura MD, Quon P, Wern Goh J, Bourret J, Peeples-Lamirande K, et al. An economic evaluation of tofacitinib for the treatment of moderately-to-severely active ulcerative colitis: modeling the cost of treatment strategies in the United States. J Med Econ. 2019;22(9):859–68. https://doi.org/10.1080/13696998.2019.1609481.
Wu B, Wang Z, Zhang Q. Cost-effectiveness of different strategies for the treatment of moderate-to-severe ulcerative colitis. Inflamm Bowel Dis. 2018;24(11):2291–302. https://doi.org/10.1093/ibd/izy114.
Sardesai A, Dignass A, Quon P, Milev S, Cappelleri JC, Kisser A, et al. Cost-effectiveness of tofacitinib compared with infliximab, adalimumab, golimumab, vedolizumab and ustekinumab for the treatment of moderate to severe ulcerative colitis in Germany. J Med Econ. 2021;24(1):279–90. https://doi.org/10.1080/13696998.2021.1881323.
Hasegawa M, Komoto S, Shiroiwa T, Fukuda T. Formal implementation of cost-effectiveness evaluations in Japan: a unique health technology assessment system. Value Health. 2020;23(1):43–51. https://doi.org/10.1016/j.jval.2019.10.005.
Guideline for Preparing Cost-Effectiveness Evaluation to the Central Social Insurance Medical Council. https://c2h.niph.go.jp/tools/guideline/guideline_en.pdf.
The Organization for Economic Co-operation and Development (OECD). Exchange rates. 2023. https://data.oecd.org/conversion/exchange-rates.htm. Accessed 8th Feb 2023
The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. https://pubmed.ncbi.nlm.nih.gov/33782057/.
Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369(8):711–21. https://doi.org/10.1056/NEJMoa1215739.
Motoya S, Watanabe K, Ogata H, Kanai T, Matsui T, Suzuki Y, et al. Vedolizumab in Japanese patients with ulcerative colitis: a Phase 3, randomized, double-blind, placebo-controlled study. PLoS One. 2019;14(2):e0212989. https://doi.org/10.1371/journal.pone.0212989.
Abridged Life Tables for Japan 2018. https://www.mhlw.go.jp/english/database/db-hw/lifetb18/index.html.
Winthrop KL, Melmed GY, Vermeire S, Long MD, Chan G, Pedersen RD, et al. Herpes zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflamm Bowel Dis. 2018;24(10):2258–65. https://doi.org/10.1093/ibd/izy131.
JMDC claims database. https://www.jmdc.co.jp/en/jmdc-claims-database/.
National Institute for Health and Care Excellence (NICE). Vedolizumab (EntyvioVR) for the treatment of adults with moderate to severe active ulcerative colitis. Single technology appraisal (STA); 2014.
Drolet M, Brisson M, Schmader KE, Levin MJ, Johnson R, Oxman MN, et al. The impact of herpes zoster and postherpetic neuralgia on health-related quality of life: a prospective study. CMAJ. 2010;182(16):1731–6. https://doi.org/10.1503/cmaj.091711.
Canadian Agency for Drugs and Technologies in Health (CADTH). CADTH COMMON DRUG REVIEW Pharmacoeconomic Review Report for Xeljanz2019 March 2019 Contract No.: Final.
Park KT, Tsai R, Perez F, Cipriano LE, Bass D, Garber AM. Cost-effectiveness of early colectomy with ileal pouch-anal anastamosis versus standard medical therapy in severe ulcerative colitis. Ann Surg. 2012;256(1):117–24. https://doi.org/10.1097/SLA.0b013e3182445321.
PFIZER. Adhoc analysis of OCTAVE Sustain (1096 DB Maintenance) [data on fle]. Study report output for PRJA392 submission (ibd_pub) Protocol (SCSA3920202a).
PFIZER. Adhoc Analysis of OCTAVE Induction 1 and 2 (1094, 1095 DB Induction); Data on file: Study Report Output for PRJA392 Submission (ibd_pub) Protocol (SCSA3920202a).
Guide to Japan’s National Health Insurance (NHI) System. https://yosida.com/forms/nationalins.pdf#:~:text=Japan%E2%80%99s%20National%20Health%20Insurance%20%28NHI%29%20system%20is%20supported,maintain%20a%20healthy%20lifestyle.%20Copayments%20made%20by%20members.
Miyazaki C, Sakashita T, Jung W, Kato S. Real-world prescription pattern and healthcare cost among patients with ulcerative colitis in Japan: a retrospective claims data analysis. Adv Ther. 2021;38(5):2229–47. https://doi.org/10.1007/s12325-020-01615-4.
American Gastroenterological Association. https://gastro.org/guidelines/.
American College of Gastroenterology. https://gi.org/guidelines/.
American College of Gastroenterology https://gi.org/guidelines/.
American Society of Colon and Rectal Surgeons. https://ascrs.org/advocacy/regulatory/guidelines.
European Crohn's and Colitis Organisation. https://www.ecco-ibd.eu/publications/ecco-guidelines-science/published-ecco-guidelines.html.
Taxonera C, de Andrés-Nogales F, García-López S, Sánchez-Guerrero A, Menchén B, Peral C, et al. Cost-effectiveness analysis of using innovative therapies for the management of moderate-to-severe ulcerative colitis in Spain. Expert Rev Pharmacoecon Outcomes Res. 2022;22(1):73–83. https://doi.org/10.1080/14737167.2021.1880324.
Essat M, Tappenden P, Ren S, Bessey A, Archer R, Wong R, et al. Vedolizumab for the treatment of adults with moderate-to-severe active ulcerative colitis: an evidence review Group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2016;34(3):245–57. https://doi.org/10.1007/s40273-015-0334-3.
Tofacitinib for previously treated active severe ulcerative colitis ID1218. https://www.nice.org.uk/guidance/ta547/documents/final-matrix.
Stern S, Ward AJ, Saint-Laurent Thibault C, Camacho F, Rahme E, Naessens D, et al. Cost-effectiveness of golimumab for the treatment of patients with moderate-to-severe ulcerative colitis in Quebec using a patient level state transition microsimulation. J Med Econ. 2018;21(1):27–37. https://doi.org/10.1080/13696998.2017.1371033.
Yokomizo L, Limketkai B, Park KT. Cost-effectiveness of adalimumab, infliximab or vedolizumab as first-line biological therapy in moderate-to-severe ulcerative colitis. BMJ Open Gastroenterol. 2016;3(1):e000093. https://doi.org/10.1136/bmjgast-2016-000093.
Reinisch W, Sandborn WJ, Hommes DW, D’Haens G, Hanauer S, Schreiber S, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60(6):780–7.
Sandborn WJ, Van Assche G, Reinisch W, Colombel JF, D’Haens G, Wolf DC, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142(2):257–65 (e3).
Suzuki Y, Motoya S, Hanai H, Matsumoto T, Hibi T, Robinson AM, et al. Efficacy and safety of adalimumab in Japanese patients with moderately to severely active ulcerative colitis. J Gastroenterol. 2014;49(2):283–94.
Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353(23):2462–76.
Jiang X-L, Cui H-F, Gao J, Fan H. Low-dose infliximab for induction and maintenance treatment in Chinese patients with moderate to severe active ulcerative colitis. J Clin Gastroenterol. 2015;49(7):582–8.
Eberhardson M, Karlén P, Linton L, Jones P, Lindberg A, Kostalla MJ, et al. Randomised, double-blind, placebo-controlled trial of CCR9-targeted leukapheresis treatment of ulcerative colitis patients. J Crohns Colitis. 2017;11(5):534–42.
Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146(1):85–95.
Sands BE, Sandborn WJ, Panaccione R, O’Brien CD, Zhang H, Johanns J, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381(13):1201–14.
Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel J-F, Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699–710.
Motoya S, Watanabe K, Ogata H, Kanai T, Matsui T, Suzuki Y, et al. Vedolizumab in Japanese patients with ulcerative colitis: a phase 3, randomized, double-blind, placebo-controlled study. PLoS One. 2019;14(2): e0212989.
Sands BE, Peyrin-Biroulet L, Loftus EV Jr, Danese S, Colombel J-F, Törüner M, et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med. 2019;381(13):1215–26.
Sandborn WJ, Su C, Sands BE, D’Haens GR, Vermeire S, Schreiber S, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376(18):1723–36.
Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367(7):616–24.
Sandborn WJ, Baert F, Danese S, Krznarić Ž, Kobayashi T, Yao X, et al. Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. Gastroenterology. 2020;158(3):562–72 (e12).
Acknowledgements
The authors’ heartfelt appreciation goes to Saurabh Trikha and Vivek Rosario of IQVIA for providing medical writing support, which was funded by Pfizer Japan Inc. in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Pfizer Japan Inc. was the only direct sponsor of this study, and fees were paid to IQVIA Solutions Japan K.K.
Conflict of interest
The authors declare the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: TK received lecture fees from AbbVie GK, Activaid, Alfresa Pharma Corporation, Gilead Sciences, Nippon Kayaku Co., Ltd., Eli Lilly Japan K.K., Mochida Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, and EA Pharma. TK received consulting fees from Takeda Pharmaceutical Co., Ltd., Activaid, Alfresa Pharma Corporation, Zeria Pharmaceutical Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Mitsubishi Tanabe Pharma Corporation, AbbVie GK, Pfizer Japan Inc., Janssen Pharmaceutical K.K., Thermo Fisher Diagnostics K.K., JIMRO Co., Ltd., Bristol-Myers Squibb, Gilead Sciences, Galapagos, and Eli Lilly Japan K.K. TK received grants from Zeria Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Otsuka Holdings, EA Pharma Co., Ltd., JIMRO Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Google Asia Pacific Pte. Ltd., Alfresa Pharma Corporation, and JMDC Inc. MH is a full-time employee of Pfizer Japan Inc. AY and SA are full-time employees of Pfizer Japan Inc., and hold stocks and stock options from Pfizer Inc. MI was a full-time employee of Pfizer Japan Inc. at the time this study was conducted. HM and SWK are full-time employees of IQVIA Solutions Japan K.K. TH received honorarium from Aspen Japan K.K., AbbVie GK., Ferring, Gilead Sciences, Janssen Pharmaceutical K.K., JIMRO Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Mochida Pharmaceutical Co, Ltd., Pfizer Japan Inc., and Takeda Pharmaceutical Co., Ltd. TH received consulting fees from Apo Plus Station, AbbVie GK., Bristol-Myers Squibb, Celltrion, EA Pharma, Eli Lilly Japan K.K., Gilead Sciences, Janssen Pharmaceutical K.K., Kyorin Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Nichi-Iko Pharmaceutical, Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd., and Zeria Pharmaceutical Co., Ltd.
Ethics approval
Not applicable. This cost-effectiveness analysis was used to simulate the long-term clinical and economic results of tofacitinib and other biologics based on existing literature findings and completed clinical trials. Moreover, the current research does not involve any studies on human participants or animals directly performed by any of the authors.
Consent to participle
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Except for clinical data and other confidential data associated with modeling and data analysis, all data are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Author contributions
All named authors take responsibility for the integrity of the work as a whole and have given final approval for this version to be published. All authors conceived and designed the study, populated the model inputs, and reviewed the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kobayashi, T., Hoshi, M., Yuasa, A. et al. Cost-Effectiveness Analysis of Tofacitinib Compared with Biologics in Biologic-Naïve Patients with Moderate-to-Severe Ulcerative Colitis in Japan. PharmacoEconomics 41, 589–604 (2023). https://doi.org/10.1007/s40273-023-01254-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-023-01254-x