Abstract
Background
Migraine is a common neurological disease that disproportionately affects females and has a peak incidence during productive years, resulting in significant burden.
Objective
The aim of the study was to determine the cost effectiveness of erenumab for the preventive treatment of migraine.
Methods
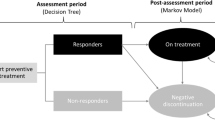
A hybrid decision-tree plus Markov model was developed to evaluate the cost effectiveness of erenumab as a migraine treatment compared with best supportive care only for patients experiencing at least 4 monthly migraine days for whom at least two prior preventive treatments had failed. Clinical efficacy data were based on results from four randomized controlled trials of erenumab against placebo. The primary outcomes were costs, migraine days, and quality-adjusted life-years (QALYs). An incremental cost-effectiveness ratio (ICER) was estimated as the cost per QALY gained. The cost per migraine day avoided was also estimated, as were disaggregated direct and indirect costs. The analysis was conducted from Swedish societal and healthcare system perspectives based on total migraine, chronic migraine and episodic migraine populations, using a discount rate of 3% applied to both costs and health benefits and using year 2019 values.
Results
In the base-case deterministic analyses, erenumab treatment resulted in ICERs of Swedish krona (SEK) 34,696 (€3310) and SEK301,565 (€28,769) per QALY gained in the total migraine and episodic migraine populations, respectively. Erenumab was dominant in the chronic migraine population. In the total migraine population, the use of erenumab resulted in a net benefit to society of SEK81,739 (€7773) per patient, assuming a willingness-to-pay threshold of SEK300,000 (€28,528) per QALY.
Conclusions
Our analysis suggests that erenumab is a cost-effective treatment for migraine with a willingness-to-pay threshold of SEK300,000 per QALY.





Similar content being viewed by others
References
Payne KA, Varon SF, Kawata AK. The International Burden of Migraine Study (IBMS): study design, methodology, and baseline cohort characteristics. Cephalalgia. 2011;31(10):1116–30.
Collaborators GBDH. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954–76.
Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21–34.
Bigal ME, Liberman JN, Lipton RB. Age-dependent prevalence and clinical features of migraine. Neurology. 2006;67(2):246–51.
Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343–9.
Dahlof C, Linde M. One-year prevalence of migraine in Sweden: a population-based study in adults. Cephalalgia. 2001;21(6):664–71.
Linde M, Stovner LJ, Zwart JA, Hagen K. Time trends in the prevalence of headache disorders. The Nord-Trondelag Health Studies (HUNT 2 and HUNT 3). Cephalalgia. 2011;31(5):585–96.
Lipton RB, Manack Adams A, Buse DC, Fanning KM, Reed ML. A comparison of the chronic migraine epidemiology and outcomes (CaMEO) study and American Migraine Prevalence and Prevention (AMPP) study: demographics and headache-related disability. Headache. 2016;56:1280–9.
Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders (ICHD), 3rd ed. Cephalalgia. 2018;38:1–211.
Evers S, Afra J, Frese A, et al. EFNS guideline on the drug treatment of migraine—revised report of an EFNS task force. Eur J Neurol. 2009;16(9):968–81.
Swedish Headache Society. Treatment guidelines migraine. 2017. http://www.huvudvarkssallskapet.se/diagnostik_behandling.php?category_id=3. Accessed Sept 2019.
British Association for the Study of Headache. National Headache Management System for Adults. 2019. http://www.bashorguk/guidelines. Accessed Sept 2019.
Linde M, Gustavsson A, Stovner LJ, et al. The cost of headache disorders in Europe: the Eurolight project. Eur J Neurol. 2012;19(5):703–11.
Olesen J, Gustavsson A, Svensson M, et al. The economic cost of brain disorders in Europe. Eur J Neurol. 2012;19(1):155–62.
Hjalte F, Olofsson S, Persson U, Linde M. Burden and costs of migraine in a Swedish defined patient population—a questionnaire-based study. J Headache Pain. 2019;20(1):65.
Selekler MH, Gokmen G, Steiner TJ. Productivity impact of headache on a heavy-manufacturing workforce in Turkey. J Headache Pain. 2013;14:88.
Hepp Z, Dodick DW, Varon SF, et al. Adherence to oral migraine preventive medications among patients with chronic migraine. Cephalalgia. 2015;35:478–88.
Vo P, Paris N, Bilitou A, et al. Burden of Migraine in Europe using self-reported digital diary data from the migraine buddy application. Neurol Ther. 2018;7(2):321–32.
Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377(22):2123–32.
Dodick DW, Ashina M, Brandes JL, et al. ARISE: a phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38(6):1026–37.
Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16(6):425–34.
EMA. Erenumab EMA summary of product characteristics. 2018. https://www.ema.europa.eu/documents/product-information/aimovig-epar-product-information_en.pdf. Accessed 26 Nov 2019.
Goadsby PJ, Paemeleire K, Broessner G, et al. Efficacy and safety of erenumab (A MG334) in episodic migraine patients with prior preventive treatment failure: a subgroup analysis of a randomized, double-blind, placebo-controlled study. Cephalalgia. 2019;39:817–29.
Ashina M, Tepper S, Brandes JL, Reuter U, et al. Efficacy and safety of erenumab (A MG334) in chronic migraine patients with prior preventive treatment failure: a subgroup analysis of a randomized, double-blind, placebo-controlled study. Cephalalgia. 2018;38(10):1611–21.
Reuter U, Goadsby PJ, Lanteri-Minet M, et al. Efficacy and tolerability of erenumab in episodic migraine patients who previously failed 2–4 preventive treatments: a randomised placebo-controlled phase 3b study. Lancet. 2018;392:2280–7.
Mahon R, Huels J, Hacking V, et al. Economic evaluations in migraine: systematic literature review and a novel approach. J Med Econ. 2020. https://doi.org/10.1080/13696998.2020.1754840.
Mahon R, Vo P, Cooney P, et al. A model concept for assessing the cost-effectiveness of preventive migraine treatments. In: Presented at: 12th European Headache Federation Congress; 28–30 September 2018; Florence, Italy.
Martelletti P, Schwedt TJ, Lanteri-Minet M, et al. My migraine voice survey: a global study of disease burden among individuals with migraine for whom preventive treatments have failed. J Headache Pain. 2018;19(1):115.
Turner IM, Newman SM, Entin EJ, Agrillo T. Prophylactic treatment of migraine with botulinum toxin type A: a pharmacoeconomic analysis in a community setting. J Med Econ. 2007;10(4):355–66.
Läkemedelsregistret National prescription registry. Analysis of 2005 to 2016 claims. https://www.socialstyrelsen.se/statistik-och-data/register/alla-register/lakemedelsregistret/. Accessed Dec 2019.
Stockholm Medical Committee. Neurology drug formulary committee Stockholm: treatment guidelines migraine. http://www.janusinfo.se/Behandling/Expertradsutlatanden/Neurologiska-sjukdomar/Anvand-anfallsforebyggande-behandling-hos-migranpatienter-med-tva-ellerfler-behandlingskravande-anfall-per-manad/. Accessed Dec 2019.
Buse DC, Lipton RB, Hallström Y, et al. Migraine-related disability, impact, and health-related quality of life among patients with episodic migraine receiving preventive treatment with erenumab. Cephalalgia. 2018;38(10):1622–31.
Sun H, Dodick DW, Silberstein S, et al. Safety and efficacy of A MG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15(4):382–90.
Ashina M, Kudrow D, Reuter U, et al. Long-term tolerability and nonvascular safety of erenumab, a novel calcitonin gene-related peptide receptor antagonist for prevention of migraine: a pooled analysis of four placebo-controlled trials with long-term extensions. Cephalalgia. 2019;39(14):1798–808.
Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–91.
https://www.scottishmedicines.org.uk/media/1356/botulinum_toxin_a_botox_2nd_resub_final_jan_2017_for_website.pdf. Accessed June 2018.
Sacco S, Ornello R, Ripa P, Pistoia F, Carolei A. Migraine and hemorrhagic stroke a meta-analysis. Stroke. 2013;44(11):3032–8.
Ashina M, Dodick D, Goadsby PJ, et al. Erenumab (A MG 334) in episodic migraine: interim analysis of an ongoing open-label study. Neurology. 2017;89(12):1237–43.
Santoro A, Fontana A, Miscio AM, Zarrelli MM, Copetti M, Leone MA. Quarterly repeat cycles of onabotulinumtoxinA in chronic migraine patients: the benefits of the prolonged treatment on the continuous responders and quality-of-life conversion rate in a real-life setting. Neurol Sci. 2017;38(10):1779–89.
NICE. The reference case. Guide to the methods of technology appraisal 2013 (P MG9). 2013. https://www.nice.org.uk/process/pmg9/chapter/the-reference-case#measuring-and-valuing-health-effects. Accessed 15 June 2017.
Rabin R, Gudex C, Selai C, Herdman M. From translation to version management: a history and review of methods for the cultural adaptation of the EuroQol five-dimensional questionnaire. Value Health. 2014;17(1):70–6.
Kosinski M, Bayliss MS, Bjorner JB, et al. A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res. 2003;12(8):963–74.
Gillard PJ, Devine B, Varon SF, Liu L, Sullivan SD. Mapping from disease-specific measures to health-state utility values in individuals with migraine. Value Health. 2012;15(3):485–94.
Hatswell AJ, Pennington B, Pericleous L, Rowen D, Lebmeier M, Lee D. Patient-reported utilities in advanced or metastatic melanoma, including analysis of utilities by time to death. Health Qual Life Outcomes. 2014;12:140.
NICE. NICE-TA350. Secukinumab for treating moderate to severe plaque psoriasis. 2015. https://www.nice.org.uk/guidance/ta350. Accessed 26 June 2017.
Blumenfeld AM, Varon SF, Wilcox TK, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS). Cephalalgia. 2011;31(3):301–15.
Berra E, Sances G, De Icco R, et al. Cost of chronic and episodic migraine. A pilot study from a tertiary headache centre in northern Italy. J Headache Pain. 2015;16:532.
Batty AJ, Hansen RN, Bloudek LM, et al. The cost-effectiveness of onabotulinumtoxinA for the prophylaxis of headache in adults with chronic migraine in the UK. J Med Econ. 2013;16(7):877–87.
Doane MJ, Vo P, Bilitou A, Fang J, Laflamme K, Gupta S. Economic impact of migraine in the EU5: a matched analysis of the NHWS 2017 data on work productivity and healthcare resource use. J Headache Pain. 2018; 19. Poster presented at the 12th European Headache Federation (EHF) Congress, 28–30th September 2018, Florence, Italy.
Södra regionvårdsnämnden. Regionala priser och ersättningar för Södra sjukvårdsregionen. 2019. http://sodrasjukvardsregionen.se/avtal-priser/regionala-priser-och-ersattningar/. Accessed Dec 2019.
Tandvårds- och läkemedelsförmånsverket. Periodens vara. 2019. https://www.tlv.se/apotek/utbyte-av-lakemedel-pa-apotek/periodens-varor.html. Accessed Dec 2019.
Messali A, Sanderson JC, Blumenfeld AM, et al. Direct and indirect costs of chronic and episodic migraine in the United States: a web-based survey. Headache. 2016;56(2):306–22.
Stewart WF, Lipton RB, Kolodner KB, Sawyer J, Lee C, Liberman JN. Validity of the migraine disability assessment (MIDAS) score in comparison to a diary-based measure in a population sample of migraine sufferers. Pain. 2000;88(1):41–52.
Statistics Sweden. Inkomst av tjänst (antal personer, medelvärden och totalsumma) efter region, kön, ålder och inkomstklass. År 2000–2016. 2018. http://www.statistikdatabasenscbse/pxweb/sv/ssd/START__HE__HE0110__HE0110A/InkAvTjanst/?rxid=f45f90b6-7345-4877-ba25-9b43e6c6e299. Accessed Dec 2019.
Swedish Tax Agency (Skatteverket). Arbetsgivaravgift. 2018. https://www.skatteverketse/foretagorganisationer/arbetsgivare/socialavgifter/arbetsgivaravgifter4233f91f71260075abe8800020817html. Accessed Dec 2019.
Statistics Sweden. Dödstal, per 1000 av medelfolkmängden efter ålder, kön och år. downloaded on 21-03-2018. http://www.statistikdatabasen.scb.se/pxweb/sv/ssd/START__BE__BE0101__BE0101I/Dodstal/. Accessed Dec 2019.
Dental and pharmaceutical benefits agency (TLV). General guidelines for economic evaluations. 2003. http://www.tlv.se/Upload/English/Guidelines-for-economic-evaluations-LFNAR-2003-2.pdf. Accessed 11 May 2015.
Fenwick E, Byford S. A guide to cost-effectiveness acceptability curves. Br J Psychiatry. 2005;187:106–8.
Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ. 1997;6(4):327–40.
NHWS. Burden of migraine in europe from the patients’ perspective: data on file (2017 data). Basel: Novartis; 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was sponsored by Novartis Pharma AG.
Conflicts of Interest
Ronan Mahon, Andrea Lang, Jasper Huels, Philip Cooney, Andriy Danyliv, Umakanth Vudumula, Sreelatha Vadapalle and Pamela Vo are employees of Novartis. Peter J. Goadsby has received personal fees from Alder Biopharmaceuticals, Allergan, Autonomic Technologies Inc., Biohaven Pharmaceuticals Inc., Clexio, Electrocore LLC, eNeura Inc, Impel Neuropharma, MundiPharma, Novartis, Teva Pharmaceuticals and WL Gore; grants and personal fees from Amgen and Eli Lilly and Company; a grant from Celgene; and other funding from Trigemina, all outside the submitted work; has a patent magnetic stimulation for headache licensed to eNeura without fee; and has received fees for publishing from Oxford University Press, Massachusetts Medical Society, Wolters Kluwer and fees for medico-legal work. Farooq Maniyar has received personal fees from Novartis for attending advisory board meetings, key opinion leader meetings and delivering lectures.
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Authors’ contributions
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for this version to be published.
Acknowledgements
The authors gratefully acknowledge David Campbell of Xcenda for his editorial contributions to this work.
Ethics Approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mahon, R., Lang, A., Vo, P. et al. Cost-Effectiveness of Erenumab for the Preventive Treatment of Migraine in Patients with Prior Treatment Failures in Sweden. PharmacoEconomics 39, 357–372 (2021). https://doi.org/10.1007/s40273-020-00996-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-020-00996-2